Fig. 23.1
AP (a) and lateral (b) view radiographs show 10-month-old infant with fibular hemimelia, concurrent congenital femoral deficiency, a typical anterior bow of the tibia, and equinovalgus foot deformity. AP (c) and lateral (d) view radiographs show a 2-year-old child with fibular hemimelia and congenital femoral deficiency as well as mild genu valgus and ankle valgus. AP (e) and lateral (f) view radiographs of a 12-year-old patient with fibular hemimelia who has genu valgus, mild leg length discrepancy, a ball-and-socket ankle joint, and talocalcaneal coalition. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
The treatment of FH changes according to the severity of deformity and the associated skeletal manifestation with a broad spectrum of choices: from simple contralateral epiphysiodesis in mild cases to limb reconstruction and lengthening in more severe cases, the latter composed of multiple surgical procedures during the patient’s childhood and adolescence. The common alternative to extensive lengthening is amputation and prosthetic rehabilitation [5, 8–16]. The challenge in limb lengthening is to achieve normal limb alignment and length with a functional, painless, plantigrade foot. This undertaking is often characterized by repeated surgical procedures and a high rate of complications and sequelae such as pin tract infections, residual LLD, delayed union, ankle and knee stiffness, refracture, knee subluxation, and residual foot deformities. Deciding which treatment to apply (lengthening versus amputation) can be challenging for the surgeon and the family [8–12].
Parents must be aware of all the options and the associated potential complications before making treatment decisions. Fortunately, very little can be done during the first year of life in most cases (unless the child has an associated club foot), so there is ample time to meet with the parents, discuss the options, and have them carefully consider which option is best for their family and their child.
The aim of this chapter is to discuss in detail the principles and techniques of management of lengthening reconstruction for fibular hemimelia by understanding and addressing each deformity in this complex, multifaceted disorder. A detailed description of the amputation options (Syme or Boyd) are covered in the amputation chapter (Chap. 13) by J.A. Herring.
Box 23.1. Typical Deformities Associated with FH
Tibial shortening
Equinovalgus foot
Missing all or part of fibula
Missing one or more lateral rays
Talocalcaneal coalition
Anteromedial bowing of tibia
Cruciate ligament insufficiency
Genu valgum
Hypoplasia of lateral femoral condyle
Limb length difference
Syndactyly
Ball-and-socket ankle joint
Equinovarus foot
Classification
Several classifications exist for fibular hemimelia. The earliest published classification is the Coventry and Johnson [17] classification from 1952 (Fig. 23.2). They divided patients with FH into three types:
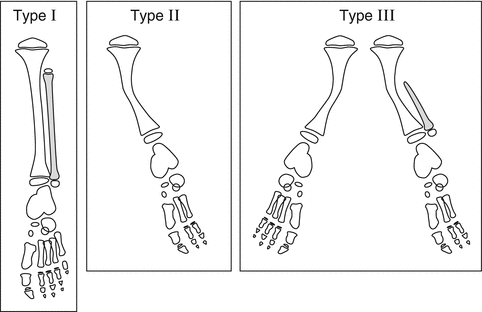

Fig. 23.2
Coventry and Johnson classification of FH: Type I: partial unilateral absence of fibula, normal or slight bowing of the tibia with some shortening of the limb, and the foot is normal or slightly deformed. Type II: fibula is completely or almost entirely absent, anterior bowing of the tibia with skin dimpling, deformed ankle joint, and deformed foot with absent rays. Type III: includes type I or II associated with other congenital deformities or bilateral involvement. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
Type I: Partial unilateral absence of fibula; normal or slight bowing of the tibia with some shortening of the limb; the foot is normal or slightly deformed.
Type II: The fibula is completely or almost absent; anterior bowing of the tibia with skin dimpling; deformed ankle joint; deformed foot with absent rays.
Type III: Includes type I or type II but is associated with other congenital deformities or bilateral involvement.
The Achterman and Kalamchi classification, from 1979 [18], is the most commonly used (Fig. 23.3). They divided the patients into two groups with one subdivision:
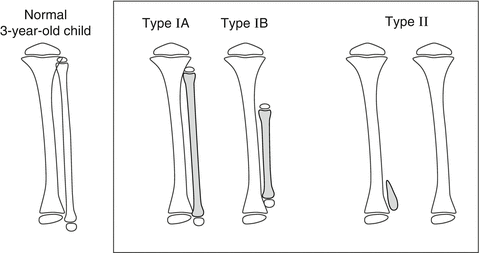

Fig. 23.3
Achterman and Kalamchi classification of FH: Type I: incomplete fibular deficiency, subdivided into I-A and I-B. Type I-A: proximal fibular epiphysis is distal to the level of the tibial growth plate, distal fibular growth plate is proximal to the dome of the talus. Type I-B: 30–50 % partial fibular absence, fibula does not articulate with the talus. Type II: complete fibular deficiency, with or without a tiny distal fibular remnant. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
Type I: Incomplete fibular deficiency; Type I is subdivided into: Type I-A: The proximal fibular epiphysis is distal to the level of the upper tibial growth plate and the distal fibular growth plate is proximal to the dome of the talus; and Type I-B: 30–50 % fibular shortening and the fibula does not articulate with the talus.
Type II: Complete fibular deficiency, with or without a small distal fibular remnant.
The Stanitski classification, from 2003 [19] (Fig. 23.4), is a morphologic description that divides FH into four categories based primarily on radiographic presentation:
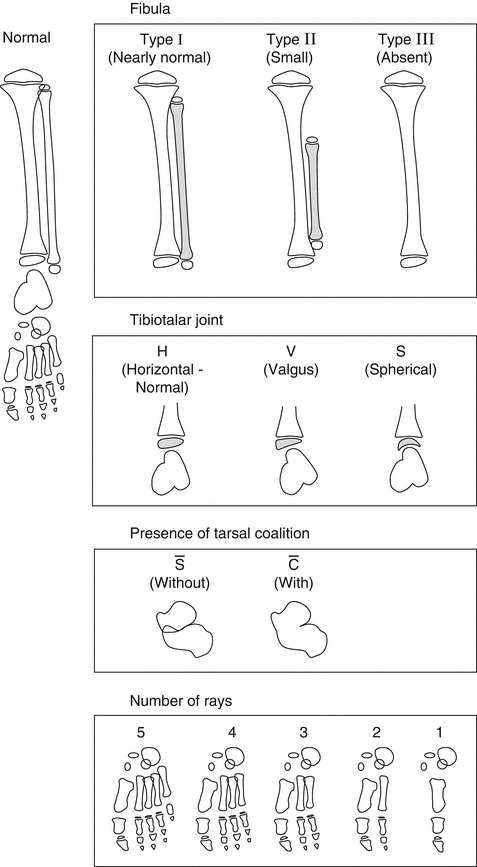

Fig. 23.4
Stanitski classification of FH according to four parameters: 1. Fibula: normal, partially absent, or completely absent. 2. Ankle joint: according to the morphology of the tibiotalar joint: horizontal, valgus, or spherical (ball-and-socket joint). 3. Tarsal bones: tarsal coalition present or absent. 4. Foot: numbers of rays. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
1.
Fibula: Normal, partially absent, or completely absent.
2.
Ankle joint: According to the morphology of the tibiotalar joint: horizontal, valgus, or ball-and-socket joint.
3.
Tarsal bones: Tarsal coalition present or absent.
4.
Foot: Numbers of rays.
Birch et al. [20] in 1998 presented a new classification system based on the clinical status of the foot and the magnitude of limb shortening as a percentage of the contralateral limb on radiographs in order to anticipate the extent of deformity at maturity and to recommend the appropriate treatment required (Fig. 23.5). In 2011, Birch later [5, 21] modified the treatment proposed: fewer amputations and more reconstruction procedures. Cases are divided into two categories:
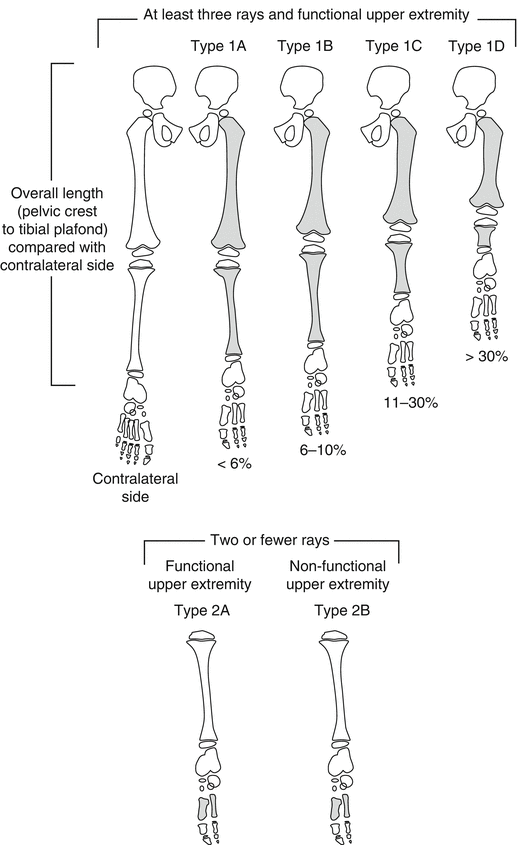

Fig. 23.5
Birch classification of FH. Type 1: Functional foot, foot with at least three rays that can provide a stable weight-bearing surface. Type 1 is divided into four subtypes according to the limb length difference compared with the normal side. Type 1A: <6 % limb length inequality; Type 1B: 6–10 % limb length inequality; Type 1C: 11–30 % limb length inequality; Type 1D: >30 % limb length inequality. Type 2: Nonfunctional foot (fewer than three rays). Type 2 is subdivided into Types 2A and 2B according to the functionality of the upper extremity. Type 2A: Functional upper extremity; Type 2B: Nonfunctional upper extremity. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
Type 1: Functional foot, in which the foot has at least three rays and can provide a stable weight-bearing platform. Type 1 is divided into four subtypes according to the percentage of total limb shortening compared with the contralateral side.
Type 1A: <6 % leg length inequality; the treatment is orthosis or contralateral epiphysiodesis.
Type 1B: 6–10 % leg length inequality; the treatment is epiphysiodesis ± lengthening.
Type 1C: 11–30 % leg length inequality; the treatment is one or two lengthening procedures ± epiphysiodesis or extension orthosis.
Type 1D: >30 % leg length inequality; the treatment proposed is more than two lengthening procedures or amputation or extension orthosis.
Type 2: Nonfunctional foot. Type 2 is subdivided into Types 2A and 2B according to the functionality of the upper extremity.
Type 2A: Upper extremities are functional; therefore, the treatment proposed is early amputation.
Type 2B: Upper extremities are nonfunctional, and so foot amputation is contraindicated, as the foot must act as a replacement for the upper extremity.
Box 23.2. Classification Schemes Used in North America for FH
Coventry and Johnson
Achterman and Kalamchi
Stanitski
Birch
Paley
A more recent classification proposed by Paley [16] is surgically oriented (reconstruction, not amputation) (Fig. 23.6). He classified FH into four types, based on the ankle pathology:
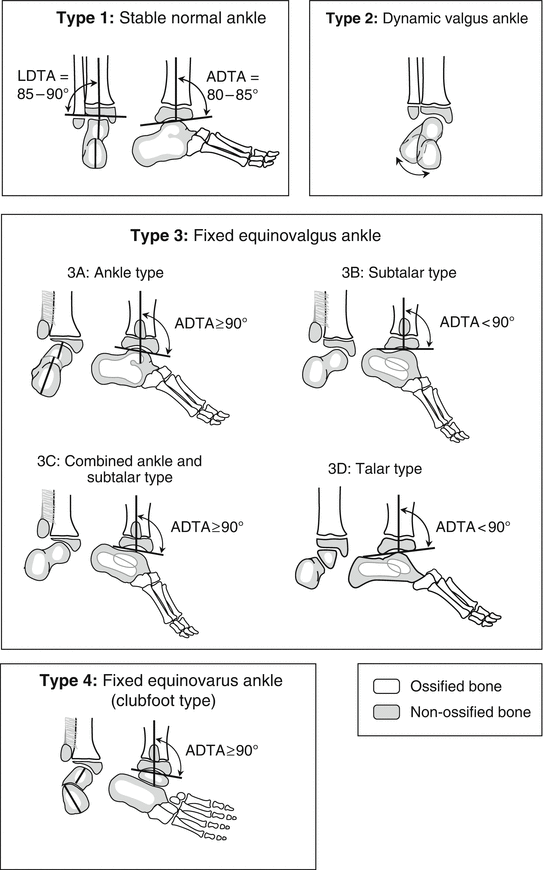

Fig. 23.6
Paley classification of FH. Type 1: Stable normal ankle. Type 2: Dynamic valgus ankle. Type 3: Fixed equinovalgus ankle. Type 3 is further subdivided according to the etiology of the valgus into 3A (ankle), 3B (subtalar), 3C (combined ankle and subtalar), and 3D (talar). Type 4: Fixed equinovarus ankle (clubfoot). Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
Type 1: Stable normal ankle.
Type 2: Dynamic valgus ankle.
Type 3: Fixed equinovalgus ankle. Type 3 is divided into four subtypes according to the location of the valgus deformity.
Type 3a: Ankle type.
Type 3b: Subtalar type.
Type 3c: Combined ankle and subtalar type.
Type 3d: Talar body type.
Type 4: Fixed equinovarus ankle (clubfoot).
For Type 1, tibial lengthening is recommended, along with tendo Achilles lengthening. For Type 2, tibial lengthening is recommended, along with tendo Achilles lengthening and a supramalleolar reorientation osteotomy. For Type 3, soft tissue lengthening (peroneal tendons and tendo Achilles), resection of the fibrous anlage and interosseous membrane, and a reorientation osteotomy is recommended. This has been nicknamed the “Super-ankle procedure” and will be described later in the chapter. The osteotomy site varies according to whether the deformity is classified as Type 3a, 3b, 3c, or 3d. Type 3a needs a supramalleolar osteotomy, type 3b needs a subtalar osteotomy, Type 3c needs both supramalleolar and subtalar osteotomy, and Type 3d, which is very rare, may be treated with an opening wedge osteotomy of the body of the talus. Type 4 (clubfoot type) is treated initially by applying Ponseti casts and performing an Achilles tenotomy followed by a “Super-ankle procedure” at age 12–24 months. The casting treatment typically converts the foot position from equinovarus to equinovalgus, which can then be addressed with the Super-ankle procedure. While the more severe Type 3 cases should also be considered candidates for amputation, Paley’s classification is geared towards reconstruction, with the subtype designation dictating the specific reconstructive procedure.
Box 23.3. Paley Classification of FH
Type 1: Normal ankle
Type 2: Dynamic valgus ankle
Type 3a: Fixed equinovalgus ankle, ankle type
Type 3b: Fixed equinovalgus ankle, subtalar type
Type 3c: Fixed equinovalgus ankle, combined ankle/subtalar type
Type 3d: Fixed equinovalgus ankle, talar body type
Type 4: Equinovarus type (clubfoot)
Clinical Assessment of a Child with FH
In our practice, a patient with fibular hemimelia is usually seen during the first year of life. In less than one-third of cases, the diagnosis has already been made using prenatal ultrasonography [22]. During the initial visit, full-length standing anteroposterior (AP) and lateral view radiographs are obtained to evaluate the exact configuration of the skeletal anatomy of the lower legs, to determine the amount of LLD, and to look for concurrent deformities such as congenital femoral deficiency. The child’s height predication based on the long limb should be recorded. Based on the radiographic LLD (or the clinically measured LLD), the ultimate LLD at maturity can be predicted. We use the Multiplier Method [23, 24] to predict the ultimate LLD and ultimate overall height (based on the long leg) at skeletal maturity. This is easily done with the Multiplier mobile app (available for free on the Apple iTunes store for the Apple iOS platform and Google Play for the Android platform).
These two predictions (LLD and height) allow for formulation of a multistage treatment plan for the family. The family should leave the first visit with a clear picture of the pros and cons of the two main treatment arms: amputation versus reconstruction. Knowing the predicted adult height (based on the current length of the long leg) and the predicted LLD at skeletal maturity allows the surgeon to propose various treatment strategies. For example, if the Multiplier Method predicts an adult height for a boy to be 5′7″ (170 cm) with an LLD of 6 cm, then most families would choose limb lengthening as opposed to epiphysiodesis. However, if the same boy had a predicted height of 6′2″ (188 cm) with an LLD of 6 cm, then epiphysiodesis would become a more attractive option. For the reconstruction option, the family needs to be told if it will be necessary to perform foot/ankle reconstruction and how many lengthening procedures are anticipated. External fixation is the mainstay of lengthening in young children with FH [21]. In skeletally mature patients, it is possible to use the newer magnetic telescopic intramedullary (IM) nails for tibial lengthening [25, 26]. Combinations of lengthening and epiphysiodesis can also be done to minimize the number of lengthening procedures required.
The initial clinical exam should include a complete orthopedic examination of the child to look for associated anomalies. In the lower extremity, the number of rays and position of the foot and ankle are noted. Specifically, the ankle is examined to determine if it is mobile and well aligned or contracted into equinovalgus or equinovarus. The range of motion and stability of the hip, knee, and ankle are assessed. The knee joint is often somewhat unstable (ACL or PCL deficiency) and may be in excessive valgus alignment.
If the involvement of the hip and knee is severe (as in combined FH and congenital femoral deficiency), then the overall reconstruction plan is either concurrent hip/knee reconstruction or sequential reconstruction. Most commonly, the significantly involved hip and knee would be addressed first between the ages of 18 and 24 months (this is discussed at length in Chap. 22). The ankle reconstruction would then typically occur 6–12 months after the hip and knee reconstruction, although in some cases, it may be feasible to perform simultaneous hip, knee, and ankle reconstruction (Super-hip, Super-knee, and Super-ankle procedures).
Even if the femur is not involved or minimally involved, there still may be instability of the knee due to an absent ACL. In such cases, the preoperative full-length standing lateral view radiograph shows anterior subluxation of the tibia on the femur (Fig. 23.8). Consideration should be given to perform an intra-articular ACL substitution procedure prior to tibial lengthening. If the PCL is also deficient, then an extra-articular PCL reconstruction should be performed at the same time.
Radiographic Assessment of a Child with FH
The initial radiographic assessment should include plain films: full-length standing AP view radiograph depicting both legs with a lift under the short leg (Fig. 23.7) and a full-length standing lateral view of the affected leg with the knee in maximum extension (Fig. 23.8). The typical appearance in severe cases is a mild valgus deformity of the knee secondary to a hypoplastic lateral femoral condyle. This creates a mechanical lateral distal femoral angle (mLDFA) of less than 85°. Additional valgus may come from an apex anteromedial bow in the tibia. A dimple on the skin is usually visible over the apex of this bow (Fig. 23.9). A lateral view radiograph of the foot may show overlap of the talus and calcaneus or a talo-calcaneal tarsal coalition. If the talus and calcaneus are on top of one another, the ankle valgus is typically from a tilt in the distal tibial plafond. The talus and calcaneus may be stacked on top of each other on the lateral view, which points toward the ankle as the source of valgus (Fig. 23.10). If the talus and calcaneus are overlapped on the lateral view (Fig. 23.11) or side by side on the AP ankle (Fig. 23.12) view giving the appearance of a “double barrel shot gun,” then the valgus component is more likely subtalar in origin. Most patients with FH have a talo-calcaneal coalition, which may not be evident on the initial radiographs obtained during infancy (Figs. 23.13 and 23.14) but become more obvious as the coalition ossifies with age.
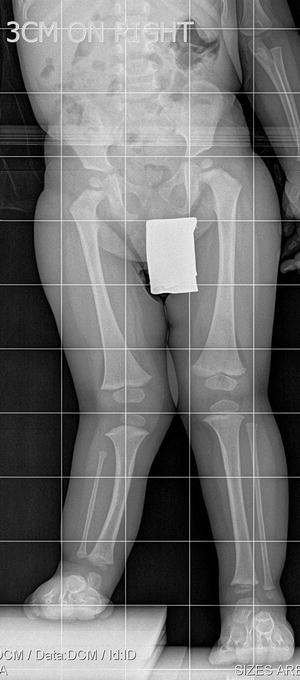
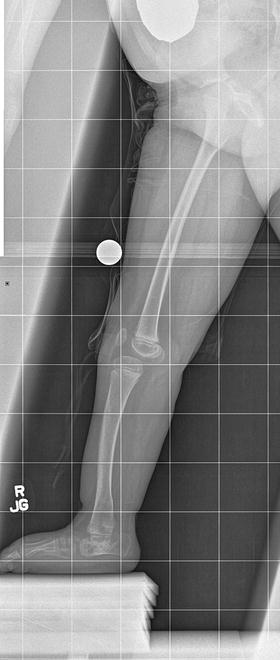
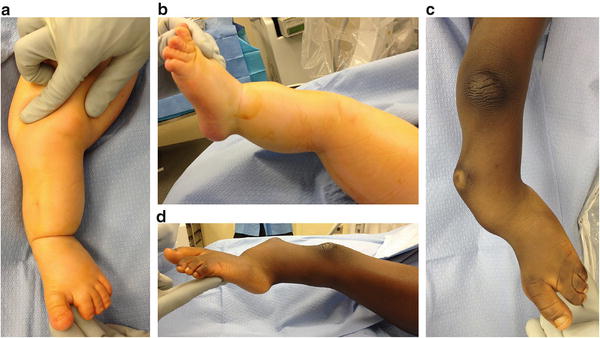
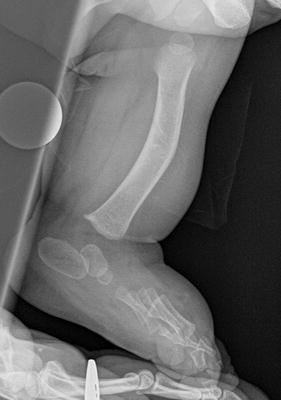
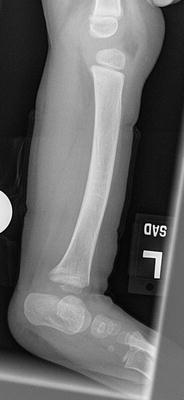
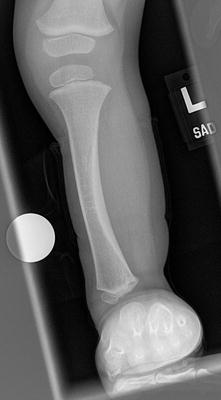
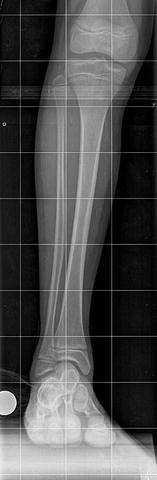
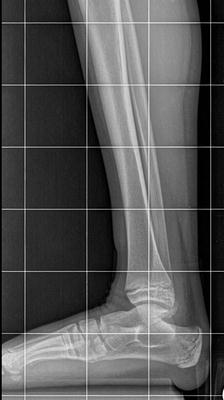

Fig. 23.7
Full-length standing AP view radiograph shows a typical child with FH who has knee valgus and tibial valgus bow. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.8
Full-length standing lateral view radiograph shows a typical child with FH who has an apex anterior bow of the tibia and congenital instability of the knee due to the lack of functional intra-articular ligaments (ACL/PCL). Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.9
(a, b) Clinical photos show a child with moderate deformity secondary to FH. The typical anterolateral bow, equinovalgus foot, and dimple at the apex of the tibial bow are shown. (c, d) Clinical photos show a child with severe deformity secondary to FH. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.10
Lateral view radiograph of the foot shows the talus and calcaneus stacked on top of each other, suggesting the hindfoot valgus is supramalleolar in origin. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.11
Lateral view radiograph of the foot shows the talus and calcaneus overlapped on top of each other, suggesting the hindfoot valgus is subtalar in origin. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.12
AP view ankle radiograph shows the talus and calcaneus side by side, suggesting the hindfoot valgus is subtalar in origin. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.13
AP view radiograph of the tibia, ankle, and foot in a typical child with FH shows a ball-and-socket ankle with a talocalcaneal coalition. As an infant, the talocalcaneal block is cartilaginous, but the talocalcaneal coalition becomes more radiographically evident as the child ages and the bones ossify. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.14
Lateral view radiograph (same child depicted in Figure 23.13) shows the classic “C” sign of the talocalcaneal coalition and a concurrent talonavicular coalition. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
In addition to plain films for cases of Paley Type 3 FH, it can be useful to obtain a magnetic resonance imaging (MRI) evaluation of the foot and ankle just prior to performing the Super-ankle procedure. Since much of the hindfoot and distal tibia is unossified during the first 2 years of life, it can be difficult to accurately classify the exact Paley Type 3 subtype. In such cases, an MRI can help differentiate between Type 3a, b, and c, thus allowing better preoperative planning (Figs. 23.15 and 23.16). Also, during surgery an arthrogram of the ankle joint can help delineate the valgus orientation at the ankle joint (Fig. 23.17).
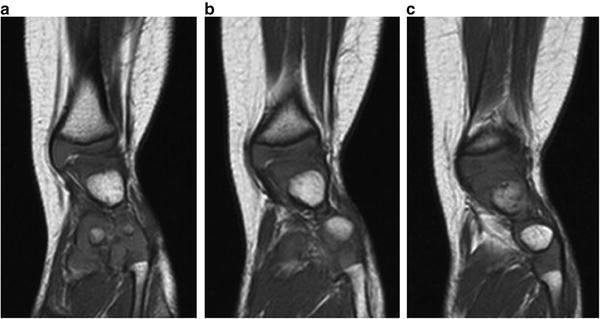
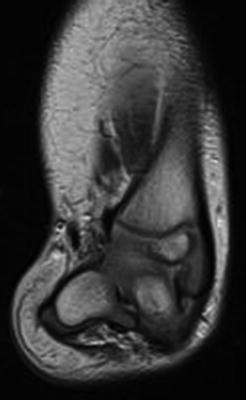
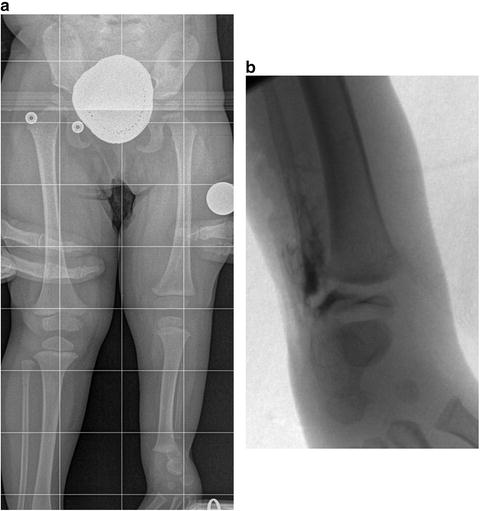

Fig. 23.15
(a–c) Sequential MRI scans of the left lower extremity in a patient with fibular hemimelia show that the valgus originates in the supramalleolar region (Paley Type 3A—ankle type). Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.16
MRI scan of the right lower extremity in a patient with fibular hemimelia shows that the hindfoot valgus originates in the subtalar joint (Paley Type 3B). Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore

Fig. 23.17
(a) Supine full-length radiograph of an 18-month-old girl with left fibular hemimelia shows typical equinovalgus of the foot and ankle. (b) Intraoperative AP ankle arthrogram of the same patient shows the distal tibia as the source of the ankle valgus. Reprinted with permission from the Rubin Institute for Advanced Orthopedics, Sinai Hospital of Baltimore
Principles of Treatment
One must understand that the initial reconstruction is a very complex set of procedures that is unique for each child. The deformity related to fibular hemimelia is the combination of contracted soft tissues and abnormally formed joints of the ankle and foot. The treatment of FH should address several issues such as limb shortening, lower leg deformity, and an abnormally positioned foot with or without absence of lateral rays. The goals of treatment are to create a lower limb with a stable hip, knee, and ankle by:
1.
Correcting the foot (usually equinovalgus) into a plantigrade position.
2.
Equalizing lower limb lengths by skeletal maturity.
3.
Correcting lower limb malalignment.
In many of the more severe cases, it may not be possible to improve the limited ankle mobility. Furthermore, the treatment itself (multiple lengthening procedures or Super-ankle reconstruction plus multiple lengthening procedures) may result in further loss of ankle motion compared to the amount present initially. The success of reconstruction is not necessarily determined by the final ankle motion. The amount of final ankle motion is usually predetermined by the amount of motion initially present at the ankle joint.
The amount of LLD predicted by the end of growth does not necessarily determine whether a successful reconstruction is possible. There are potentially no set limits to the amount of overall lengthening that can be performed, especially when divided into small steps of approximately 5 cm for each treatment. However, the overall predicted LLD does help when estimating the number of lengthening treatments that will be needed to equalize the limb lengths before skeletal maturity. Dividing the amount of lengthening into smaller amounts might be less traumatic for the muscles, nerves, and adjacent joints than trying to perform a heroic lengthening during one treatment. For example, if the predicted discrepancy is 15 cm, this may be better addressed with three 5-cm lengthening treatments instead of two 7.5-cm lengthening treatments. Better yet, two 5-cm lengthening treatments combined with one 5-cm epiphysiodesis may yield the optimum outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


