Fig. 25.1
Photograph of tibia with anterolateral bow
The pathology of CPT involves fibrous hamartoma and pathological periosteum [1]. Pathologic examination of CPT lesions has revealed highly cellular tissue similar to fibromatosis; the gap contained fibrous tissue as well as both fibrocartilage and hyaline cartilage, and was continuous with the periosteum [5]. This hamartomatous tissue is thought to interfere with bone production and callus formation, perhaps by decreasing the blood supply [2].
Associations
There is a strong association between CPT and neurofibromatosis type 1 (NF-1). Also called von Recklinghausen disease, NF-1 is inherited in an autosomal dominant manner and is relatively common, with an incidence of 1/3,000 [3]. The NF-1 gene is located on chromosome 17 and encodes the product neurofibromin [3]. As many as 75 % of CPT cases are associated with NF-1, though only 5 % of NF-1 patients develop CPT [3]. Histologically, there is no difference between cases with or without NF-1 [4]. Non-orthopedic manifestations of NF-1 include café-au-lait spots, optic gliomas, neurofibromas, malignant peripheral nerve sheath tumors, and increased risk of cardiovascular diseases [3]. Other orthopedic manifestations include scoliosis and abnormalities of the cervical spine; patients often have decreased bone mass due to the loss of neurofibromin function [3].
The method by which NF-1 is related to or causative of CPT is imperfectly understood. A recent study by DY Lee et al. suggested that the fibrous hamartoma of CPT in NF-1 results from periosteal cells that have failed in terminal osteoblastic differentiation, arresting without completing the differentiation process [6]. Another study by SM Lee et al. indicated that double inactivation of the Nf1 gene could be present in some cases of CPT but is not the essential requirement for development of CPT [7].
Pseudarthrosis of the tibia has also been described in cases of osteofibrous dysplasia or fibrous dysplasia. Boyd described several cases of CPT as having pathology similar to fibrous dysplasia in 1958 [8]; Campbell et al. reported on five cases which they termed a variant of fibrous dysplasia involving anterior bowing of the tibia [9]. Campanacci et al. reported on a series of 35 cases of what they termed osteofibrous dysplasia, one of which had a pseudarthrosis of the tibia, and ten of whom had bowing of the tibia [10].
The tibial bowing in CPT is distinctly anterolateral. Bowing in other directions occurs in rickets (typically tibia vara or valga) or in posteromedial bowing of the tibia [11] but is unrelated to pseudarthrosis of the tibia and treated very differently.
Classification Systems
Multiple classifications of CPT have been described; these include the systems of Crawford, Boyd, and Andersen.
Crawford described a classification system in 1986 based on the radiographic appearance of the tibia in neurofibromatosis; in this assessment type 4 has the worst prognosis (Fig. 25.2) [12]:
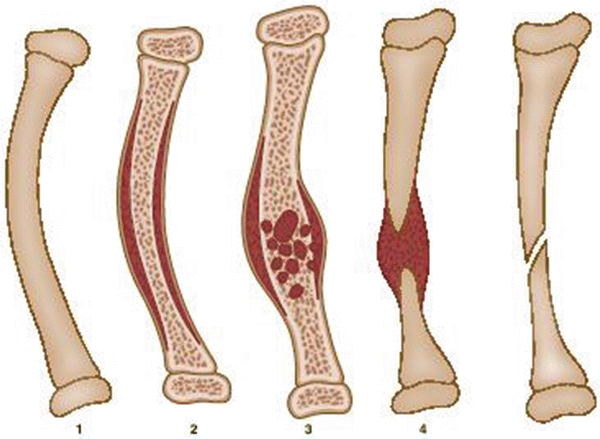

Fig. 25.2
Crawford classification of CPT: Type 1: anterolateral bow with a dense medullary canal; type 2: anterolateral bow with an increased medullary canal and a tubulation defect; type 3: anterolateral bow and a cystic lesion; type 4: anterolateral bow with evident fracture, cysts, or pseudarthrosis
Type 1: Anterolateral bow with a dense medullary canal.
Type 2: Anterolateral bow with a widened medullary canal and a tubulation defect.
Type 3: Anterolateral bow and a cystic lesion.
Type 4: Anterolateral bow with evident fracture, cysts, or pseudarthrosis.
Boyd described six types of CPT in 1982; in this assessment type 2 has the worst prognosis [13]:
Type 1: Anterior bow and defect of the tibia, possibly associated with other congenital deformities.
Type 2: Anterior bow and hourglass constriction of the tibia with fracture typically before 2 years of age; often associated with neurofibromatosis.
Type 3: CPT developing in a bone cyst, usually at the junction of the middle and lower two-thirds of the tibia.
Type 4: CPT originating in the same location without tibial narrowing but with at least partial, if not complete, narrowing of the canal.
Type 5: A dysplastic fibula with fibular or tibial pseudarthrosis.
Type 6: Intraosseous neurofibroma or Schwannoma.
Andersen described a series of 46 patients with pseudarthrosis of the leg in 1976, which he separated into six types [14]:
Club foot: True congenital fracture present at birth, associated with other congenital anomalies.
Cystic: Cystic lesions of the distal tibia and fibula without angulation or fracture at birth.
Late: Pseudarthrosis developed after fracture through a sclerotic tibia (at 4 and 9 years of age).
Fibular: Pseudarthrosis first developed in the fibula.
Dysplastic: Dysplastic, hourglass tibia with pseudarthrosis; associated w/neurofibromatosis.
Angulated: No radiographic evidence of dysplasia; pseudarthrosis developed after osteotomy; associated with neurofibromatosis.
In practice, the most relevant criterion to guide treatment is whether the tibia is fractured or intact.
Imaging
The mainstay of imaging for CPT is anterior-posterior and lateral radiographs of the affected leg. Computed tomography (CT scanning) may be used to quantify healing at the CPT site. More recently, magnetic resonance imaging (MRI) has been used to assess CPT; it has been suggested that MRI can provide additional information such as the type, length, and structure of the pseudarthrosis, as well as the extent of the affected periosteum and subtle soft-tissue changes [15]. This may assist in treatment planning and monitoring.
Prognostic Factors
Multiple prognostic factors have been proposed and debated, much of which remains controversial. Association with NF-1 was thought to indicate a poorer prognosis; however, this is no longer thought to be the case, as this has not been borne out in the literature [1]. The relationship between age and prognosis is complex. There has been debate over when to initiate surgical management, with some authors advocating early intervention and others urging waiting until at least age 4 [4]. Boyd asserted that older children would do better after surgical treatment [13]. However, Morrissy et al., reporting on a series of 40 cases of CPT, felt that older children were more likely to have a poor surgical outcome; they found that patients who still required grafting at or after age 13 years did poorly [16].
Tudisco et al. examined 30 patients with CPT who had reached the end of skeletal growth, and found that radiographic classification was correlated with prognosis. Crawford type 2 and 4 had the worst prognosis for union, with type 4 having the worst functional results [17]. Ohnishi et al., reporting on a series of 73 patients with CPT, felt that residual deformity and narrow diameter of the bone at the CPT site would lead to refracture [18].
Finally, a more distal location in the tibia may worsen the prognosis of CPT as this location is more difficult to control and necessitates the involvement of the ankle and the hindfoot in fixation [1].
Non-operative Management
In general, the treatment of CPT is surgical. Early on, patients with anterolateral bowing may be braced in order to attempt to delay progression to fracture and CPT [1]. At first, when a child is younger than 3 or 4 years old, knee-ankle-foot orthoses (KAFOs) are used in order to control rotation of the lower extremity and prevent progression to fracture . Thereafter, a patella-tendon-bearing clamshell orthosis is used with medial and lateral femoral flanges, which will also control rotation. Bracing is also frequently used postoperatively in order to support the surgical procedure.
Operative Management
CPT is a challenging disorder to treat. The underlying condition makes healing difficult, and multiple procedures and revisions may be needed. Ultimately some cases may eventually be treated with an amputation. Many procedures exist for the treatment of CPT. These include bypass grafting, intramedullary fixation, free fibula transfer, Ilizarov external fixation, periosteal grafting, Masquelet’s procedure, and amputation.
Modified McFarland Bypass Graft
Ofluoglu et al. describe a method of prophylactically treating an anterolaterally bowed tibia in NF1 with a posteromedially placed allograft fibula combined with bracing until maturity. In their series of ten, no patient had a fracture or a pseudarthrosis at final follow-up (Figs. 25.3a–c and 25.4a–f) [19].
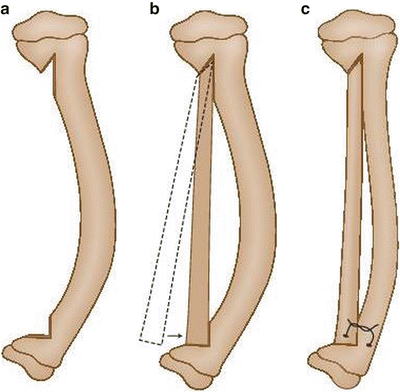
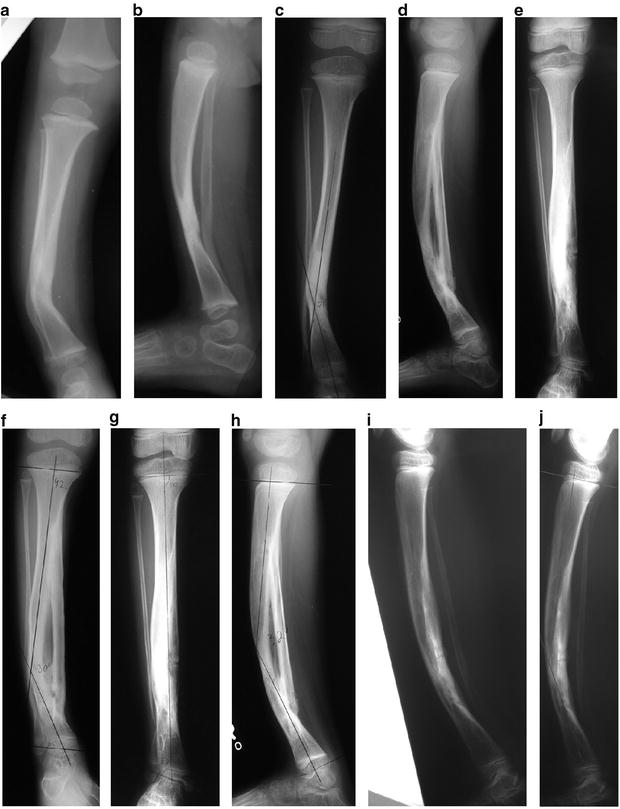

Fig. 25.3
(a–c) Modified McFarland bypass graft technique

Fig. 25.4
(a–j) CPT treated by the modified McFarland bypass graft method
This technique is performed under tourniquet with the use of an image intensifier. The patient is positioned supine and the lower leg is placed on the operating table such that the tibial deformity is in the horizontal plane. The skin and soft tissues are incised anteromedially from the proximal to the distal physis; the periosteum is then resected, taking care not to incise too close to the physes. Notches are then made in the tibial metaphysis proximally and distally about 2 cm from the physes. An allograft fibular strut, several millimeters longer than the span of the two notches, is impacted into the two notches under compression, taking care to place the graft on the concavity of the bow of the tibia to accept weight bearing. Thus, this bypasses the bowed tibia. A screw, a wire, or heavy suture is then used to fix the distal end of the graft to the tibia, and the tibia facing the graft is decorticated. Bone graft is then used to facilitate incorporation of the allograft. The patient is then placed in a long leg cast and kept non-weight bearing until radiographic evidence of bone healing is noted (6–8 weeks). Thereafter the patient wears a total contact clamshell patellar tendon-bearing orthosis (flared at the knee to control rotation) or a hinged KAFO until maturity [19]. Remodeling often leads to resorption of the tibia in favor of enlargement and incorporation of the fibular graft. Proximal and distal metaphyseal deformity may need guided growth plates and screws if remodeling is incomplete.
Intramedullary Nailing
In 1956, Charnley described the use of an intramedullary nail to treat CPT; two patients were treated with Steinmann nails in the intramedullary canal [20]. Sofield, in 1971, reported on a series of more than 100 patients with CPT, and asserted that those treated with intramedullary fixation did best; he advocated fragmentation, reversal of the fragments, and fixation with an intramedullary rod (Fig. 25.5) [21].
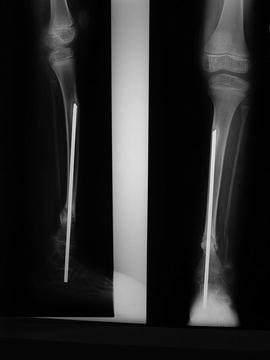

Fig. 25.5
X-rays of CPT treated with an intramedullary nail
Dobbs et al. describe a treatment method of CPT using a Peter Williams rod for stabilization after excision of the pseudarthrosis. They report a satisfactory outcome in 16 of 21 patients; complications included reoperation in 5, amputation in 5, ankle valgus in 10, and leg-length discrepancy in 11 [22].
This technique is performed under tourniquet with an image intensifier. Iliac crest bone graft is first acquired. An anterior incision is made longitudinally extending proximally and distally from the pseudarthrosis site. The pseudarthrosis is excised down to normal, bleeding medullary canal, which is then reamed both proximally and distally. A Williams rod is used to stabilize the tibia (implants such as the Fassier-Duval rod may also be considered, although there is concern for threaded screws in the growth plate). The size (length and diameter) of the rod is determined preoperatively, based on anticipated growth. In most cases, the rod traverses the ankle. In children younger than 4, it will go into the calcaneus; in patients who are 5–10 years old, the rod will end in the talus; in those older than 9 or 10 (or if the pseudarthrosis is quite proximal), the rod will not cross the ankle. The assembled rod is passed antegrade into the distal tibial segment, and then out through the talus and calcaneus, finally passing out through the heel pad, taking care to position the foot and ankle correctly in neutral. Then, the rod is passed back up retrograde into the proximal tibial segment, again taking care to align the tibia properly (this may require a second, proximal osteotomy). If the fibula is too long, it should be cut and stabilized with a Kirschner wire. The bone graft is placed around the pseudarthrosis site and secured with heavy suture. Patients are cast for about 4 months—spica initially in young children, and then above knee. Thereafter a clamshell KAFO is used with a free knee and locked ankle. In older children, this may eventually be converted to a total contact ankle-foot orthosis (AFO). Once the distal edge of the rod approaches the talar articular surface, via longitudinal growth of the tibia, a second procedure is done to advance the rod across the ankle. The orthosis is then converted to a hinged ankle and is worn until maturity [22].
Free Vascularized Fibula
A free vascularized fibular (FVF) graft from the contralateral leg may be used as a strut after excision of a tibial pseudarthrosis. This functions as a structural replacement for the excised portion of the tibia. In the ideal case, the vascularity of the graft allows it to heal more quickly, avoid resorption, and add vasculature to a scarred and dysplastic site [23]. FVF transfer requires a specialized team with microsurgery expertise, and careful attention to detail.
Several authors have reported on the results of FVF transfer in the treatment of CPT. Dormans et al. reported on a series of 12 patients in which FVF was used to treat CPT; at final follow-up, one patient had a persistent nonunion [24]. Similarly, Weiland et al. reported on a series of 19 patients with CPT treated with FVF; of these, 18 had healed at final follow-up [25]. Iamaguchi et al. present a cautionary view of this procedure; in their series of 16, 63 % required multiple surgeries to achieve union, but eventually all 16 did [26].
Ilizarov External Fixation
The Ilizarov circular external fixator may be used in CPT. Several different techniques and frame configurations have been described. Paley et al. describe a series of 15 patients with 16 CPT who were treated using the Ilizarov device. The rate of union was 94 % after one treatment and 100 % after two; there were five refractures and two residual deformities [27]. Boero et al. describe a series of 21 patients with CPT treated with the Ilizarov device; at final follow-up, 9 had consolidated without deformity or shortening, 5 consolidated with axial deviations, and 7 did not consolidate [28]. Ghanem et al. reviewed a series of 14 patients with CPT treated using the Ilizarov fixator; in all instances the pseudarthrosis site was not excised but the leg was realigned and lengthened. Union was ultimately achieved in 13 cases, 6 having required additional grafting [29] (Fig. 25.6a, b).
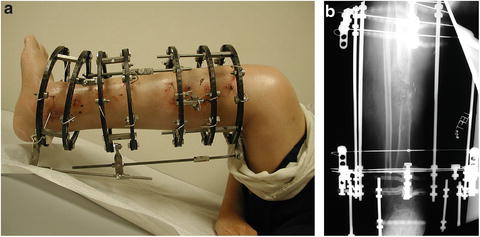

Fig. 25.6
(a, b) Clinic photo and X-ray demonstrating CPT treated with the Ilizarov device
The pseudarthrosis site may be treated by direct end-to-end compression, by side-to-side compression, or by inserting one part into another; it may also be resected, with lengthening through a proximal corticotomy, through the site itself, or by bone transport [27]. Typically, a four-ring assembly is used, with two rings proximal and two distal to the pseudarthrosis site; the foot may be included if the pseudarthrosis is very distal in the tibia [28].
Periosteal Grafting
Thabet et al. report on the results of a technique that is a combination of several methods, using intramedullary fixation and Ilizarov circular fixation along with bone and periosteal grafting. Their case series includes 20 patients, all of whom united after the first procedure; 8 patients sustained refractures, reoperation was required in 7 cases, and no amputations were performed [30].
This technique is performed under tourniquet and with an image intensifier. An anterior incision is used; the cuff of abnormal periosteum is incised, circumferentially dissected, and removed. A small longitudinal split is made in the proximal tibia segment, and the distal end is drilled open and then inserted into the proximal end. The same procedure, including periosteal excision, is performed on the fibula. Intramedullary fixation is then placed in both the tibia and fibula (static or expandable rods were both used in the referenced paper). Next, iliac crest bone graft is harvested, as well as the periosteum over the iliacus muscle. These are placed around the pseudarthrosis site, periosteum first. Finally, an Ilizarov fixator is applied, with three wires and a ring proximally and the same distally; the frame is extended to the foot [30] (Figs. 25.7a–f and 25.8a–h).
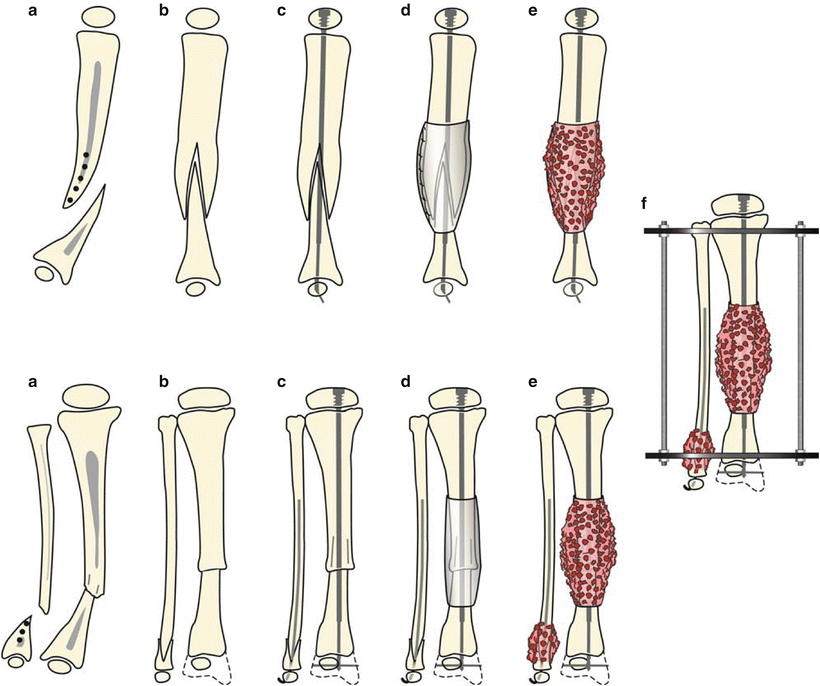

Fig. 25.7




Treatment for type I CPT in the lateral and anteroposterior (AP) views is shown. (a) There is longitudinal splitting of the proximal tibial and fibular fragments. (b) The bone ends are docked and (c) IM rods are inserted (Fassier-Duval telescopic IM nail with Paley modification illustrated) into the fibula and tibia. (d) Periosteal graft is wrapped around the pseudarthrosis and (e) iliac crest bone graft is applied to the tibial and fibular docking site. (f) External fixator is applied. With kind permission from Springer Science + Business Media: Clinical Orthopedics and Related Research, Periosteal grafting for congenital pseudarthrosis of the tibia: A preliminary report, 466(12), 2008, Thabet AM
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


