● ATRIAL SEPTAL DEFECT
Definition, Spectrum of Disease, and Incidence
The interatrial septum plays a critical role in fetal cardiac function as it provides a shunt between the right and left atria. This shunt allows the highly oxygenated umbilical venous blood to reach the coronary and cerebral circulations. An atrial septal defect (ASD) is thus a pathologic opening of the atrial septum, leading to a communication between the left and right atria. According to its embryonic origin and its location, an ASD is classified into septum primum, septum secundum, sinus venosus, and coronary sinus defect (1) (Fig. 18.1). Septum secundum defect (ASD II), which is due to lack of tissue in the region of the foramen ovale, is the most common, accounting for about 80% of all ASDs (2). Septum primum defect (ASD I), the second most common, represents a gap in the embryologic septum primum region, adjacent to both atrioventricular valves (Fig. 18.2) and is considered a partial atrioventricular septal defect (AVSD) (discussed later in this chapter). Sinus venosus ASD is found posterior and superior to the foramen ovale and inferior to the connections of the superior and inferior venae cavae within the right atrium (Fig. 18.1). The rare coronary sinus ASD is located at the site of the coronary sinus ostium in the right atrium and is often associated with coronary sinus abnormalities (Fig. 18.1). The incidence of an ASD in postnatal series is high, accounting for 7% of all infants with congenital heart defects and occurring in 1 per 1,500 live births with a 2:1 female-to-male ratio (3, 4). ASD II and sinus venosus defect are practically inexistent in prenatal series (5).
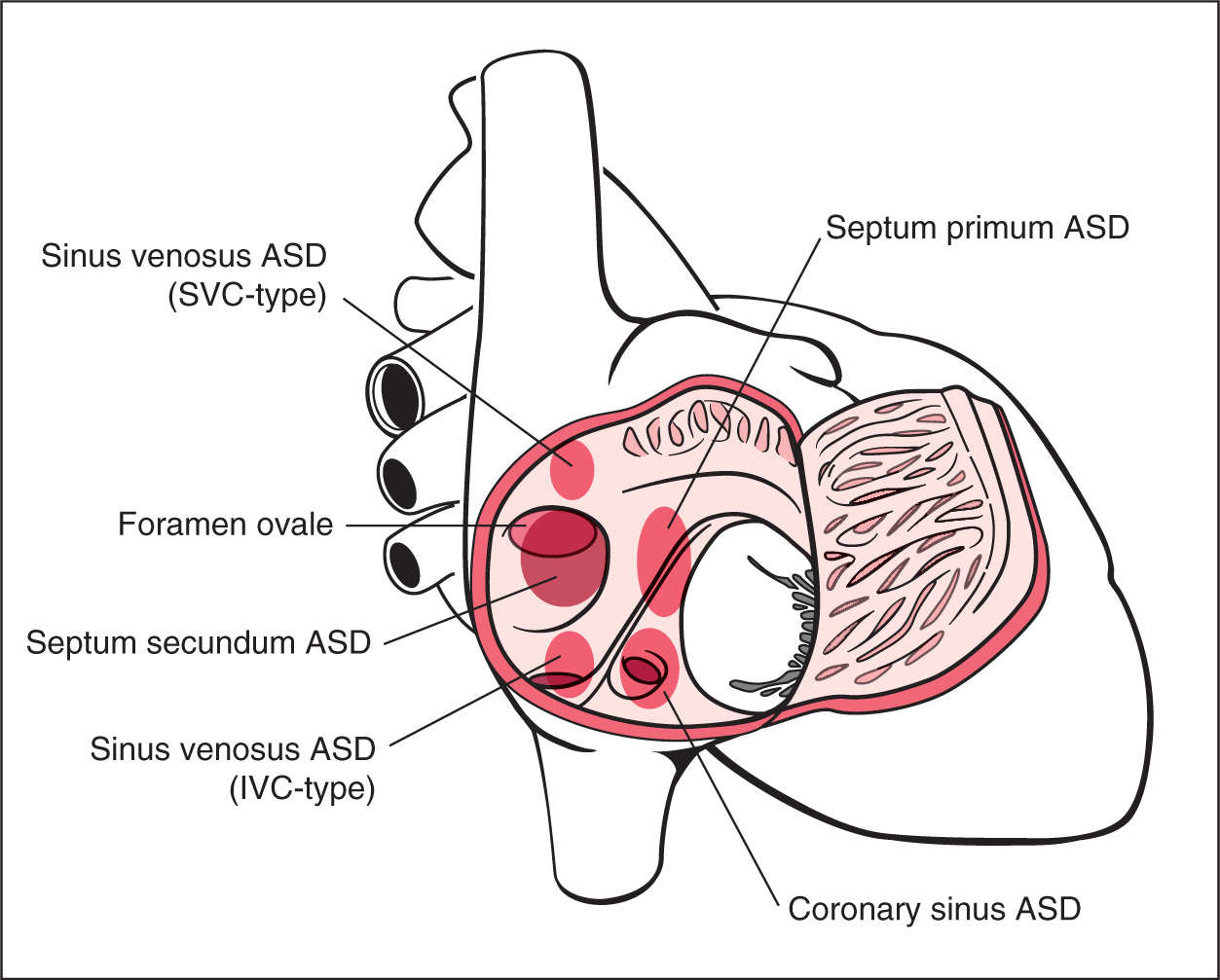
Figure 18.1: A schematic drawing showing types and anatomic locations of atrial septal defects (ASDs) as seen from an unroofed right atrium. ASDs include septum primum defect, septum secundum defect, sinus venosus defect of the superior vena cava (SVC) type and inferior vena cava (IVC) type, and coronary sinus defect. See text for details.
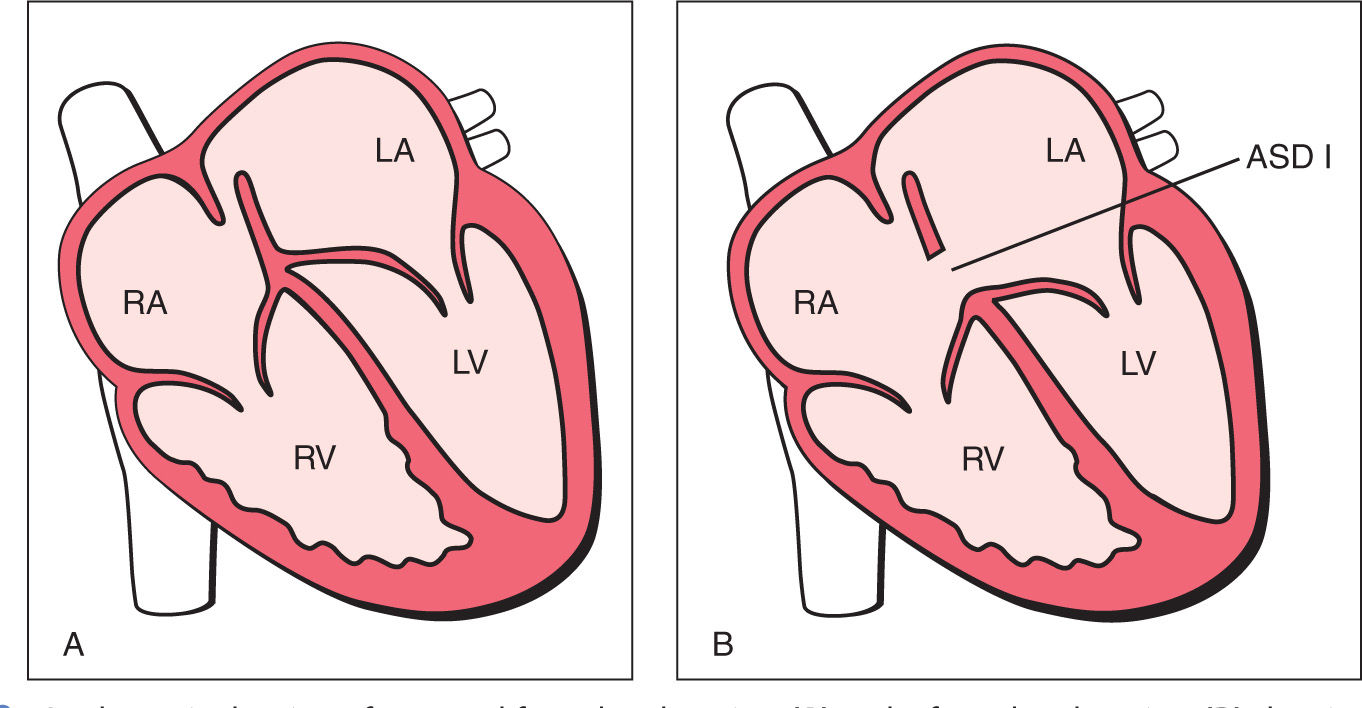
Figure 18.2: A schematic drawing of a normal four-chamber view (A) and a four-chamber view (B) showing a septum primum atrial septal defect (ASD), also known as ASD type I (ASD I), or incomplete atrioventricular septal defect. The ASD is in the septum primum region, and the atrioventricular valves show a linear insertion. LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
Ultrasound Findings
Most ASDs cannot be diagnosed in the fetus. An ASD II is located in the foramen ovale region, and whether or not a large foramen ovale will close postnatally is difficult to predict. An attempt of a “prenatal diagnosis” of ASD II is commonly associated with a high false-positive and false-negative rate. An ASD I, however, can be identified in the fetus by visualizing the gap in the septum primum region, which is generally accompanied with a linear insertion of both atrioventricular valves (Figs. 18.3 and 18.4). Color Doppler can confirm the presence of an ASD I by demonstrating a right-to-left shunting of blood across the septal defect (Fig. 18.5). These two signs are very important as the most common false-positive diagnosis can be due to the presence of a left superior vena cava draining into the dilated coronary sinus (Fig. 18.6), which mimics an ASD I (6). In the latter case, color Doppler flow is from left to right into the right atrium (Fig. 18.6). Figure 18.7 shows side by side how to differentiate with color Doppler between a septum primum defect and a dilated coronary sinus.
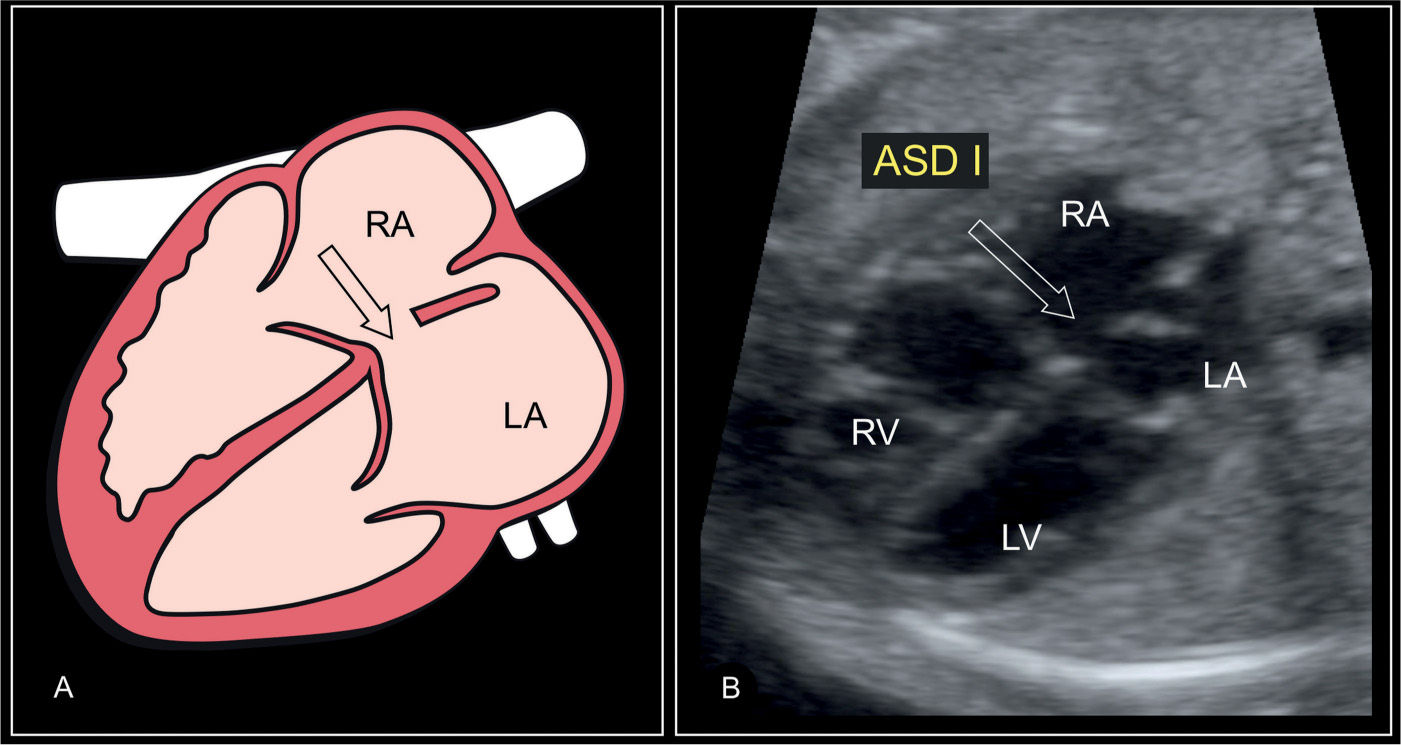
Figure 18.3: Schematic drawing and corresponding ultrasound image of a basal four-chamber view in a fetus with a primum atrial septal defect (ASD I) (arrow). Note the gap in the atrial part of the heart crux and the linear insertion of the atrioventricular valves. The remaining part of the interatrial septum and foramen ovale region appear normally developed. LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
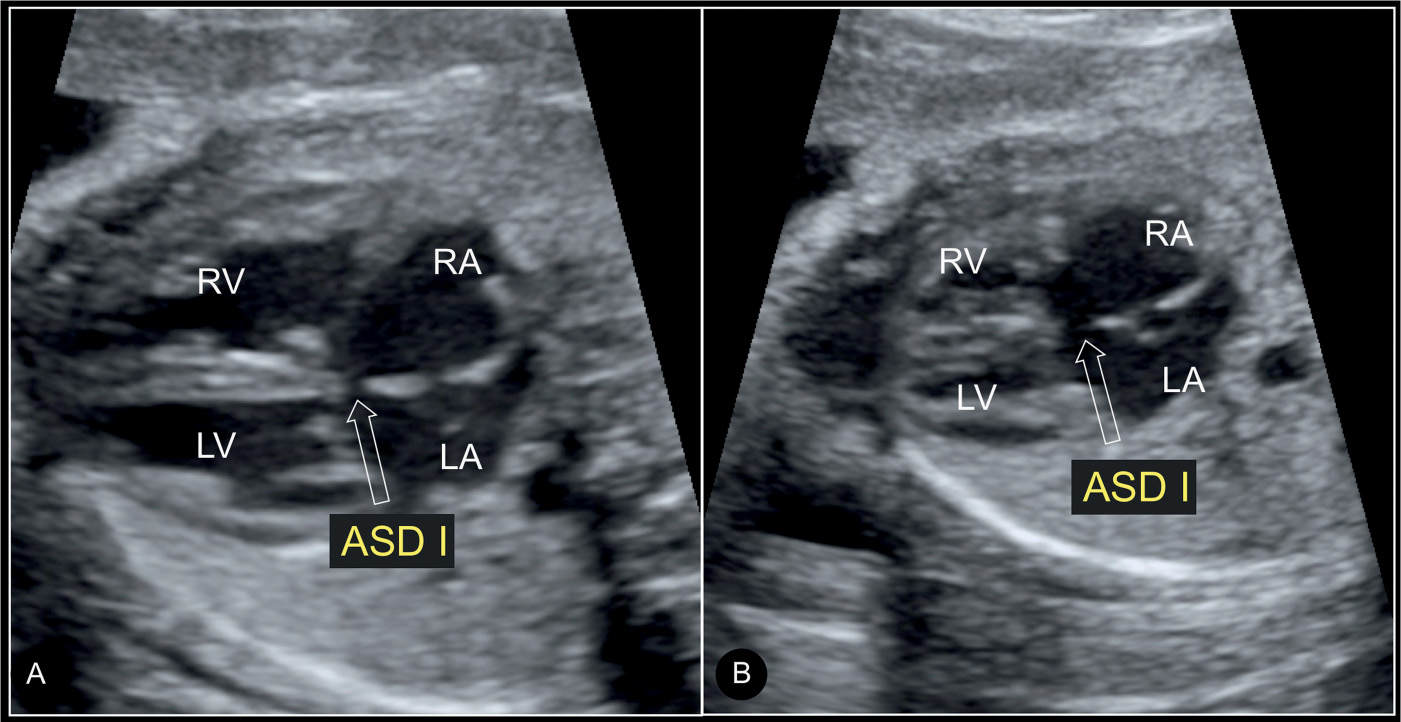
Figure 18.4: Axial four-chamber view in A (systole) and B (diastole) in a fetus with a septum primum atrial septal defect (ASD I). Note the gap in the septum primum region (open arrows) in systole (A) and diastole (B). The linear insertion of the closed atrioventricular valves is shown in systole (A) (compare with Fig. 18.3). LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
A sinus venosus defect is difficult to image even on postnatal examination. Its typical appearance could be an opening at the site of connection of the superior or inferior vena cava with the interatrial septum (Figs. 18.8 and 18.9). The image shows an override of the vein over the septal defect (Figs. 18.8B and 18.9) instead of continuity of the vein with the septum (Fig. 18.8A), best recognized in a parasagittal view. Figure 18.8A shows a normal connection of the superior vena cava with the interatrial septum and Figures 18.8B and 18.9 a sinus venosus defect with the described override.
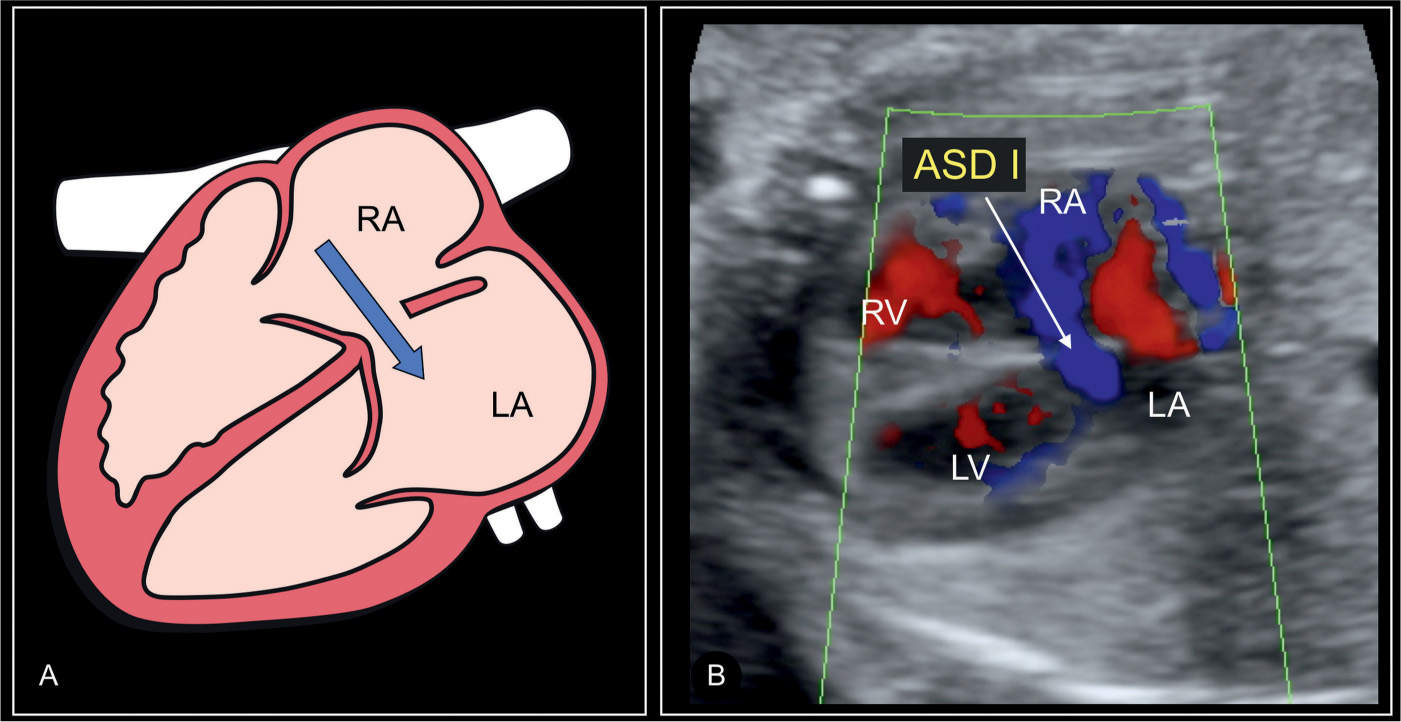
Figure 18.5: Schematic drawing (A) and corresponding color Doppler image (B) of an axial four-chamber view in a fetus with a septum primum atrial septal defect (ASD I). Note the shunting across the defect from the right atrium (RA) to the left atrium (LA) shown on color Doppler in B (solid arrow). Compare with normal coronary sinus flow in a heart with a left persistent superior vena cava, which is from the left-to-right direction as shown in Figure 18.6B. LV, left ventricle; RV, right ventricle.
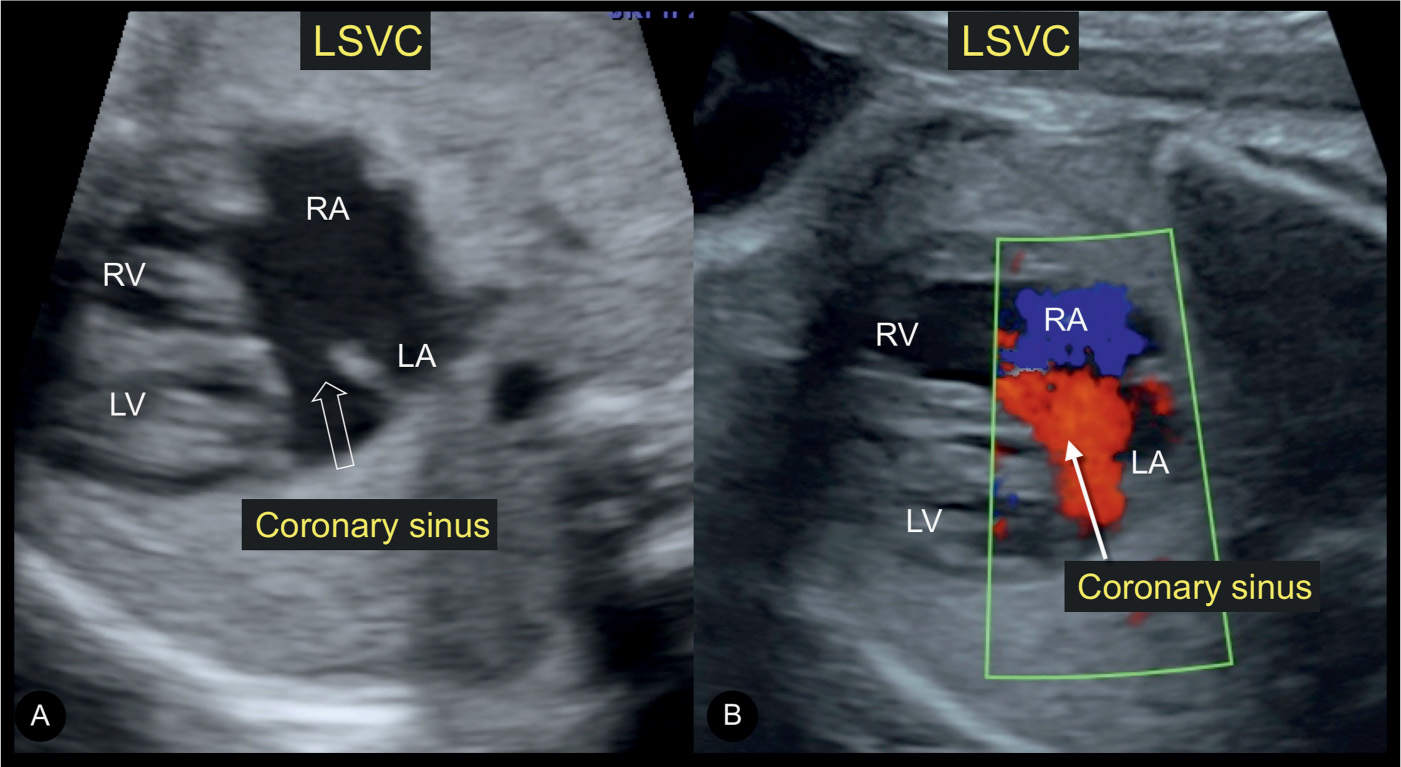
Figure 18.6: Transverse planes of the fetal chest at a level just inferior to the four-chamber in gray scale (A) and color Doppler (B) in a fetus with a persistent left superior vena cava (LSVC) and dilated coronary sinus. Note the appearance of a gap in A (open arrow), which can be misdiagnosed as a septum primum defect (ASD I) (compare with Figs. 18.3 and 18.4). Color Doppler in a dilated coronary sinus shows flow in a left-to-right direction (B), a differentiating feature from ASD I (compare with Fig. 18.4). LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
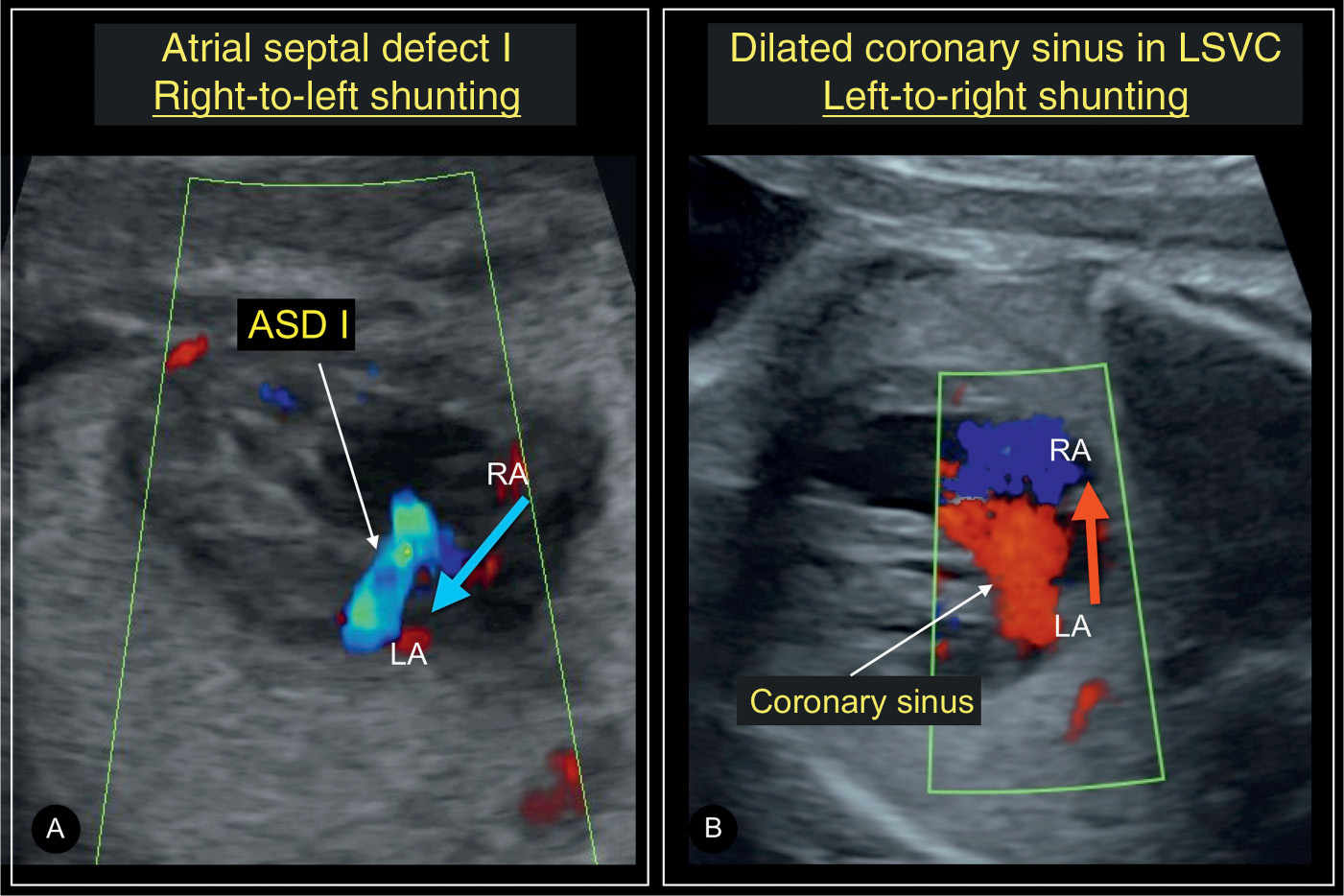
Figure 18.7: Axial four-chamber views in color Doppler in a fetus with a septum primum atrial septal defect (ASD I) (A) and another fetus with a persistent left superior vena cava (LSVC) with a dilated coronary sinus (B). Color Doppler is helpful in differentiating between ASD I and dilated coronary sinus as the grayscale appearance of these two diagnoses is quite similar. Color Doppler will demonstrate shunting from right atrium (RA) to left atrium (LA) (blue arrow) in ASD I (A), whereas the shunting is from LA to RA (red arrow) in LSVC (B). Compare with Figures 18.3B and 18.6A.
Associated Cardiac and Extracardiac Findings
ASDs are often isolated defects but prenatally detected cases are often associated with cardiac malformations, including AVSD, isomerism, abnormal pulmonary venous connection, single ventricle, or anomalies of obstructive right ventricle and outflow tract, such as Ebstein anomaly, tricuspid atresia with ventricular septal defect (VSD), and pulmonary atresia with intact ventricular septum. Abnormalities of the venous system are also common, as 10% to 15% of ASD II and 80% to 90% of the sinus venosus–superior vena cava–type defects are associated with partial anomalous venous connection (1, 7). Coronary sinus defects are commonly associated with persistent left superior vena cava.
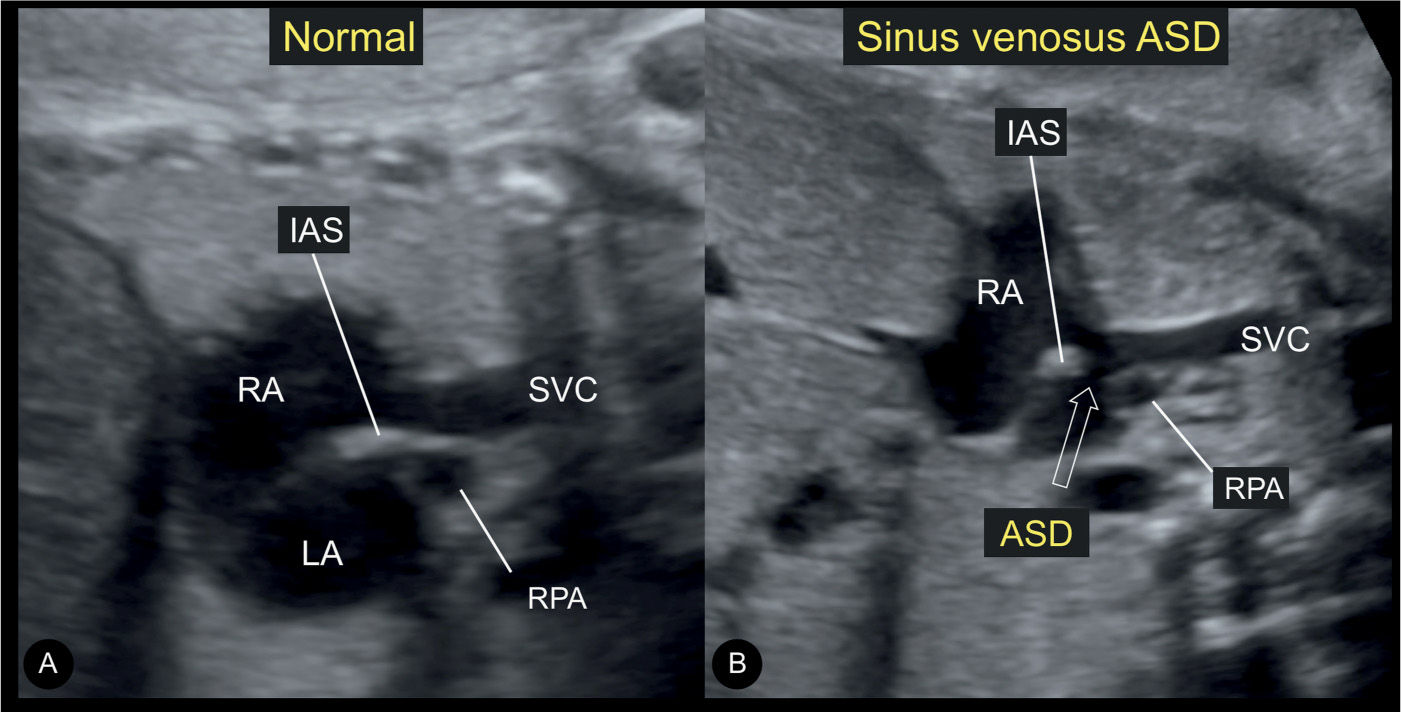
Figure 18.8: Longitudinal views on grayscale ultrasound of the junction between the superior vena cava (SVC) and the right atrium (RA) in a normal fetus (A) and a fetus with sinus venosus atrial septal defect (ASD) (B). Note the continuity between the SVC and the interatrial septum (IAS) in the normal fetus (A). In the fetus with sinus venosus ASD (open arrow in B), there is discontinuity of the SVC–IAS junction. LA, left atrium; RPA, right pulmonary artery.
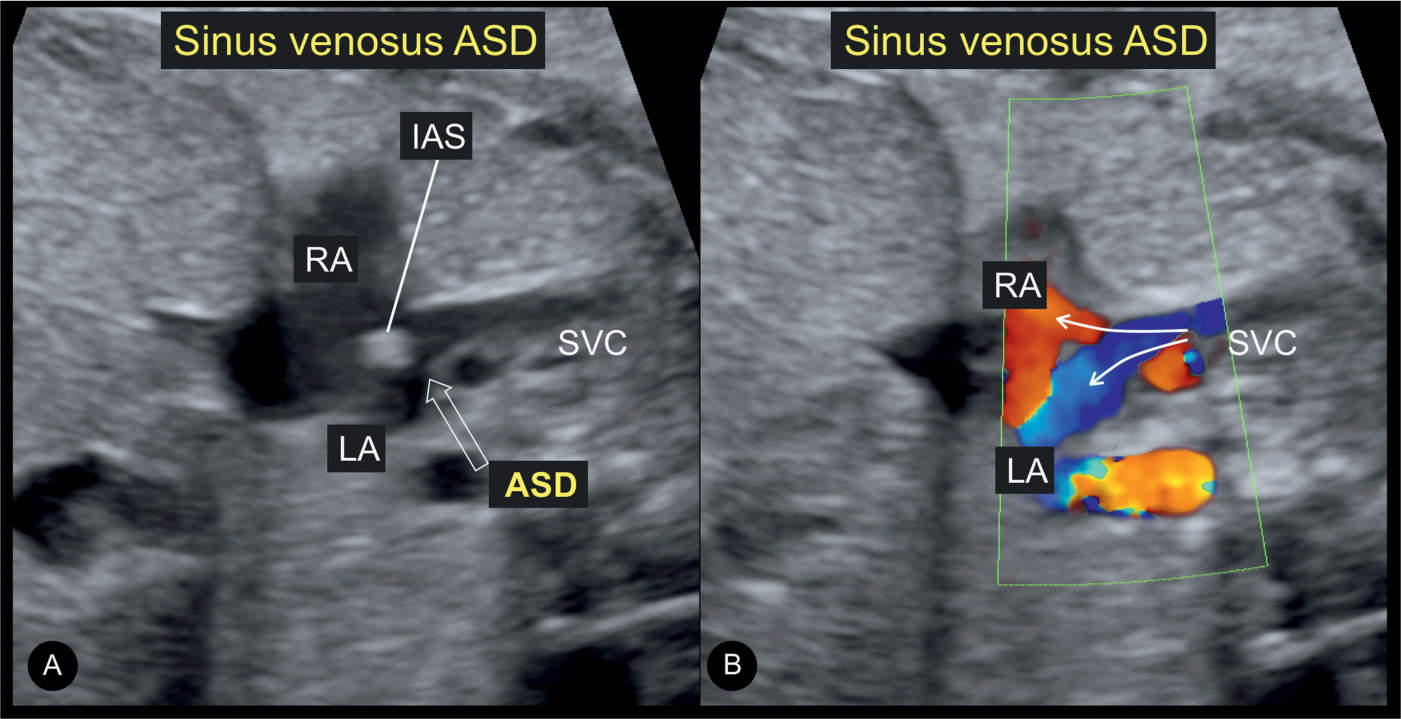
Figure 18.9: Longitudinal views on grayscale ultrasound (A) and color Doppler (B) of the same fetus as in Figure 18.8B with a sinus venosus atrial septal defect (ASD), showing the junction between the superior vena cava (SVC) and the right atrium (RA). The open arrow in A shows the ASD. Note that the SVC appears to override the ASD in A and B. Also note that color Doppler in B demonstrates blood flow streaming from the SVC into both the right (RA) and left (LA) atria (solid arrows). IAS, interatrial septum.
The ASD I, as a partial form of AVSD (pAVSD), characterized by separate atrioventricular valvular orifices with an atrial septum primum, is associated with trisomy 21, albeit to a lesser extent than the complete form. Prenatal series have shown a 12.5% association with trisomy 21, and an adjusted overall rate of aneuploidy of about 29% (6). Aortic coarctation represents the most frequently associated cardiac lesion, seen in 13% of pAVSD cases. Extracardiac anomalies are also common and are present in up to 33% of prenatally diagnosed pAVSD (6).
ASDs are described in numerous extracardiac anomalies and syndromes, such as trisomy 21 (6). Since their detection as an isolated finding is unlikely prenatally, the authors will not enumerate all possible associated syndromes but will mention in particular Holt–Oram syndrome, which is associated with an 85% to 95% risk of cardiac anomalies, most commonly ASD II and muscular VSDs (see Chapter 4).
Differential Diagnosis
Once an ASD is suspected, associated cardiac findings should be ruled out, mainly the presence of an associated AVSD or a single ventricle. A common reason for referral with a false-positive diagnosis of ASD is the presence of a dilated coronary sinus with a persistent left superior vena cava associated with left-to-right atrial shunting on color Doppler (Fig. 18.7). Furthermore, in few fetuses, referred with ectopic beats, the foramen ovale flap may appear redundant, previously described as “aneurysm of the foramen ovale” (8, 9) (Fig. 18.10), and this may be confused with an ASD.
Prognosis and Outcome
Postnatally, some small ASDs may be followed up conservatively given a significant chance for spontaneous closure, especially for the ASD II type. Moderate to large ASD II and other forms require closure in order to avoid left-to-right blood shunting with volume overload of the right atrium and subsequently the lungs. Closure is achieved either surgically or with nonsurgical techniques, such as percutaneous catheter closure devices. Short- and long-term outcomes are excellent for all forms when isolated. Prognosis of ASD associated with other cardiac malformations or genetic syndromes is dependent primarily on the severity of the associated abnormality.
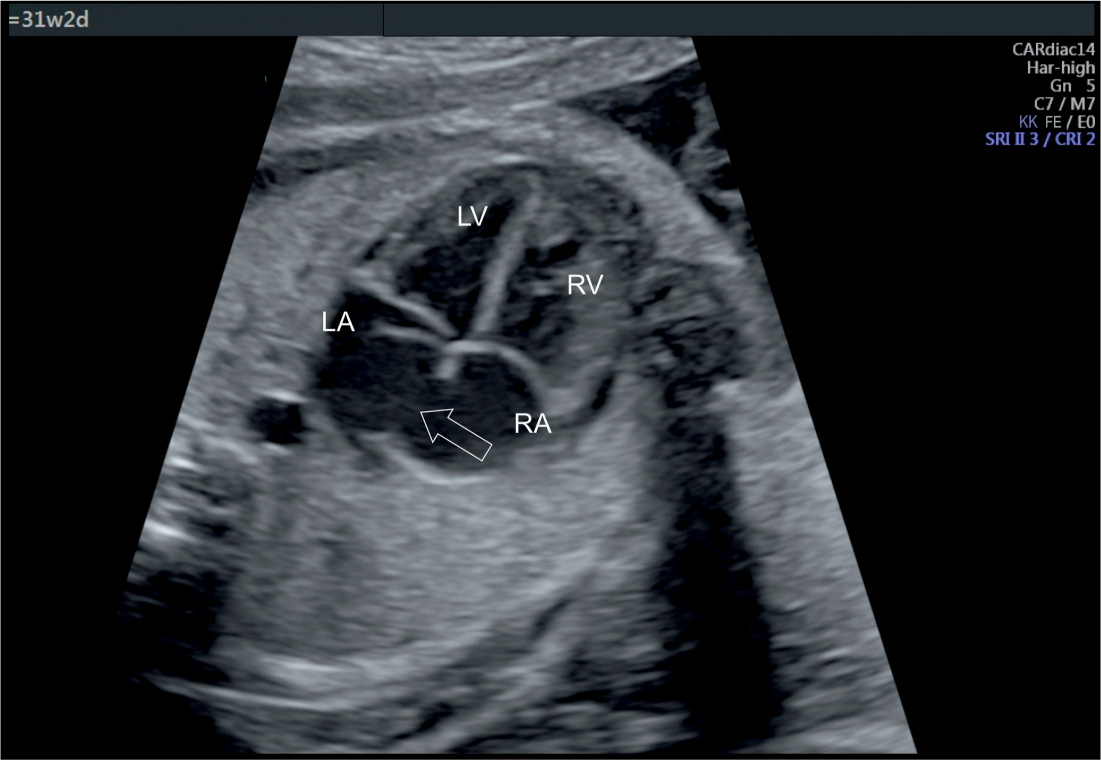
Figure 18.10: Apical four-chamber view in gray scale in a fetus with redundant flaps of the foramen ovale (open arrow). This represents a normal, isolated finding and should not be mistaken for an atrial septal defect. See text for details. LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
KEY POINTS  Atrial Septal Defects
Atrial Septal Defects
 ASDs are classified into septum primum, septum secundum, sinus venosus, and coronary sinus defects based on their anatomic locations.
ASDs are classified into septum primum, septum secundum, sinus venosus, and coronary sinus defects based on their anatomic locations.
 Septum secundum defect is the most common, accounting for about 80% of all ASDs.
Septum secundum defect is the most common, accounting for about 80% of all ASDs.
 The diagnosis of an isolated ASD is very difficult or impossible in the fetus.
The diagnosis of an isolated ASD is very difficult or impossible in the fetus.
 Septum primum ASD is called partial AVSD and is detectable by the defect in the septum primum region and the linear insertion of the atrioventricular valves.
Septum primum ASD is called partial AVSD and is detectable by the defect in the septum primum region and the linear insertion of the atrioventricular valves.
 Septum primum ASD is commonly associated with aneuploidies, such as trisomy 21.
Septum primum ASD is commonly associated with aneuploidies, such as trisomy 21.
 Partial anomalous venous connection is associated with 10% to 15% of ASD II and 80% to 90% of the sinus venosus–superior vena cava–type ASD.
Partial anomalous venous connection is associated with 10% to 15% of ASD II and 80% to 90% of the sinus venosus–superior vena cava–type ASD.
 Coronary sinus defects are commonly associated with persistent left superior vena cava.
Coronary sinus defects are commonly associated with persistent left superior vena cava.
● VENTRICULAR SEPTAL DEFECT
Definition, Spectrum of Disease, and Incidence
A ventricular septal defect (VSD) is an opening in the ventricular septum, leading to a hemodynamic communication between the left and right ventricles. VSDs are common congenital heart defects, second only to bicuspid aortic valve (10). Isolated VSDs account for 30% of children born with congenital heart defects and are associated with other cardiac anomalies in about 30% of cases (10–12). Postnatal echocardiographic evaluation reports the prevalence of VSD to be as high as 50 per 1,000 live births (13). Four anatomic components of the ventricular septum exist (14): The inlet septum separates the two atrioventricular valves, the outlet septum includes the conal and infundibular septum and relates to the region below the arterial valves and above the crista supraventricularis, the membranous septum is the small thin region in the left ventricular outflow tract just beneath the aortic valve and under the crista supraventricularis, and the thick trabecular (or muscular) septum is the largest part of the septum, which extends from the attachments of the tricuspid valves to the apex (14). Many classifications of VSDs have been proposed, and typically VSDs are reported based on their anatomic locations on the septum (Figs. 18.11 and 18.12). Perimembranous VSDs are the most common in neonatal series, accounting for about 80% of cases (10, 15). Muscular, inlet, and outlet VSDs account for 5% to 20%, 5% to 8%, and 5% of the remainder of cases, respectively (10, 15, 16) (Table 18.1). In prenatal series, however, muscular VSDs are most common and account for about 80% to 90%, with perimembranous VSDs, being the second most common (17) (Table 18.1). VSDs are frequently associated with various cardiac anomalies, as they are obligatory in some and occasionally or frequently found in others (12) as shown in Table 18.2. VSDs tend to have a high recurrence rate and are slightly more common in girls (18). Figure 18.13 shows two anatomic specimens of fetal hearts with VSDs.
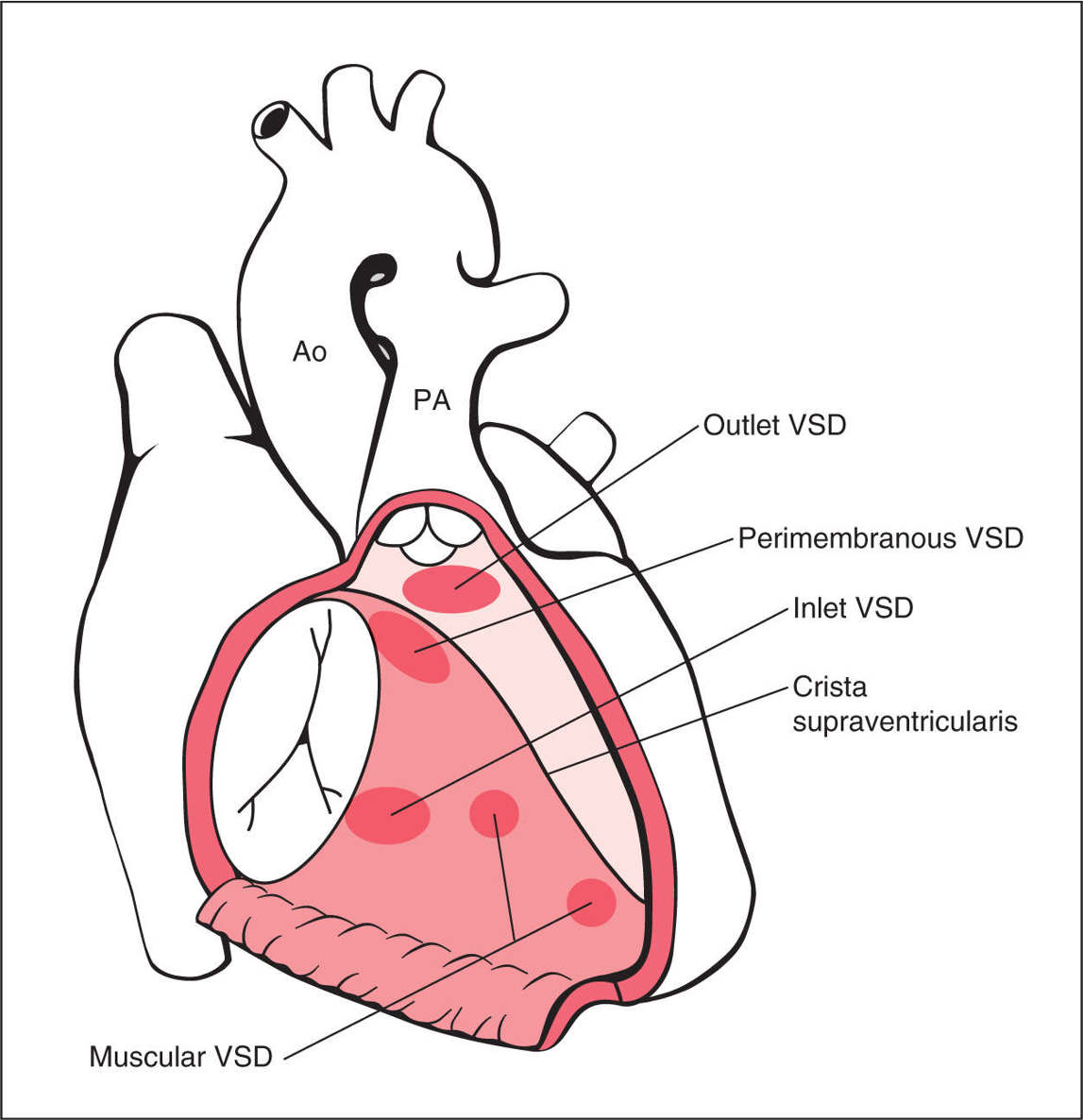
Figure 18.11: Schematic drawing of types and anatomic locations of ventricular septal defects (VSDs) as seen from an unroofed right ventricle. PA, pulmonary artery; Ao, aorta.
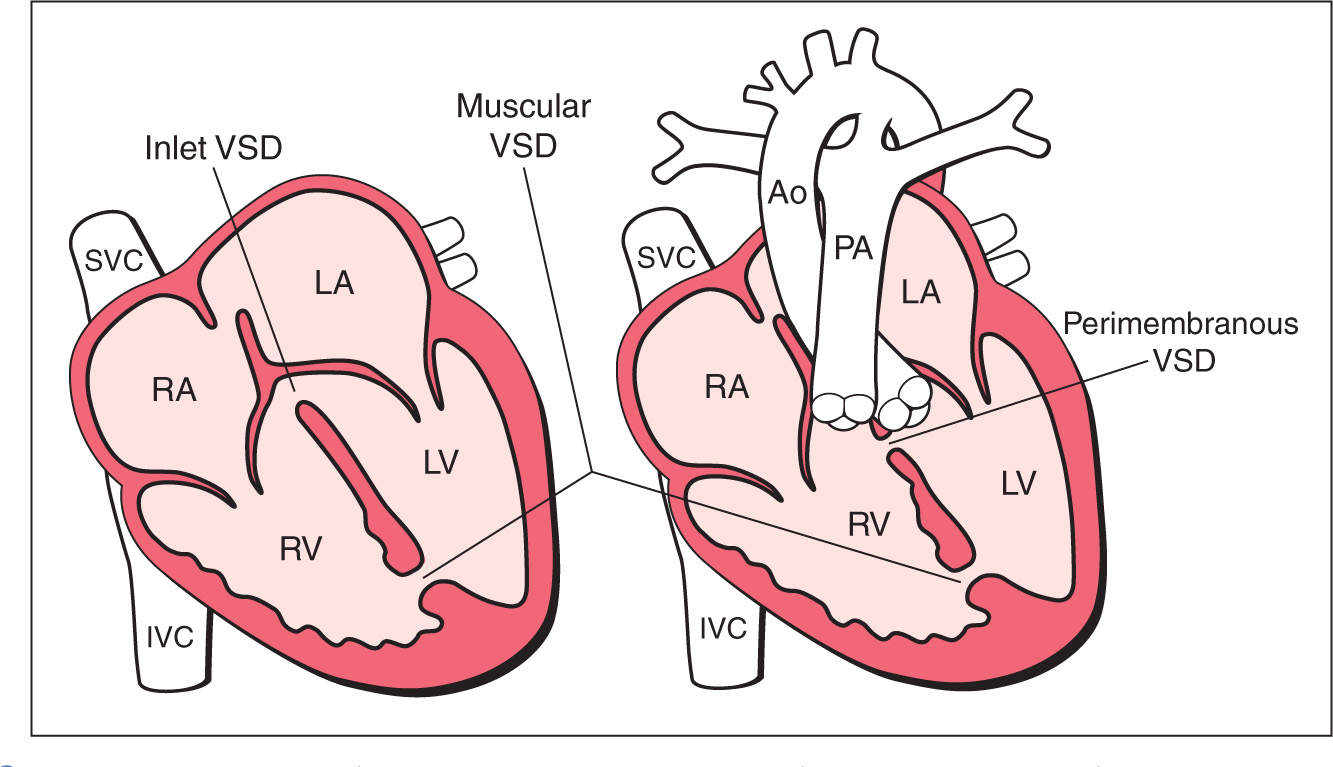
Figure 18.12: Schematic drawings of types and anatomic locations of ventricular septal defects (VSDs) as seen from the four-chamber and outflow tract views. Ao, aorta; RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; PA, pulmonary artery; SVC, superior vena cava; IVC, inferior vena cava.
Type | Also called | Location |
Perimembranous VSD | Infracristal, conoventricular | In the outflow tract beneath the aortic valve and under the supraventricular crest |
Subclassified as: perimembranous inlet perimembranous outlet perimembranous muscular | ||
Outlet VSD | Supracristal, subpulmonic, subarterial, doubly committed | Under the pulmonary valve and above the supraventricular crest |
Muscular VSD | Trabecular | Can be apical, midmuscular, or multiple (called Swiss cheese septum) |
Inlet VSD | Posterior, atrioventricular septum type | Posterior to the septal leaflet of the tricuspid valve |
Cardiac Anomalies Associated with Ventricular Septal Defects (VSDs) |
Type of association | Cardiac anomaly |
Obligatory association | Atrioventricular septal defect Tricuspid atresia + VSD Mitral atresia + VSD Single ventricle (double inlet ventricle) Tetralogy of Fallot Pulmonary atresia with VSD Absent pulmonary valve syndrome Common arterial trunk Double outlet right ventricle Interrupted aortic arch |
Occasional association | D-transposition of the great arteries Corrected transposition of the great arteries Aortic coarctation |
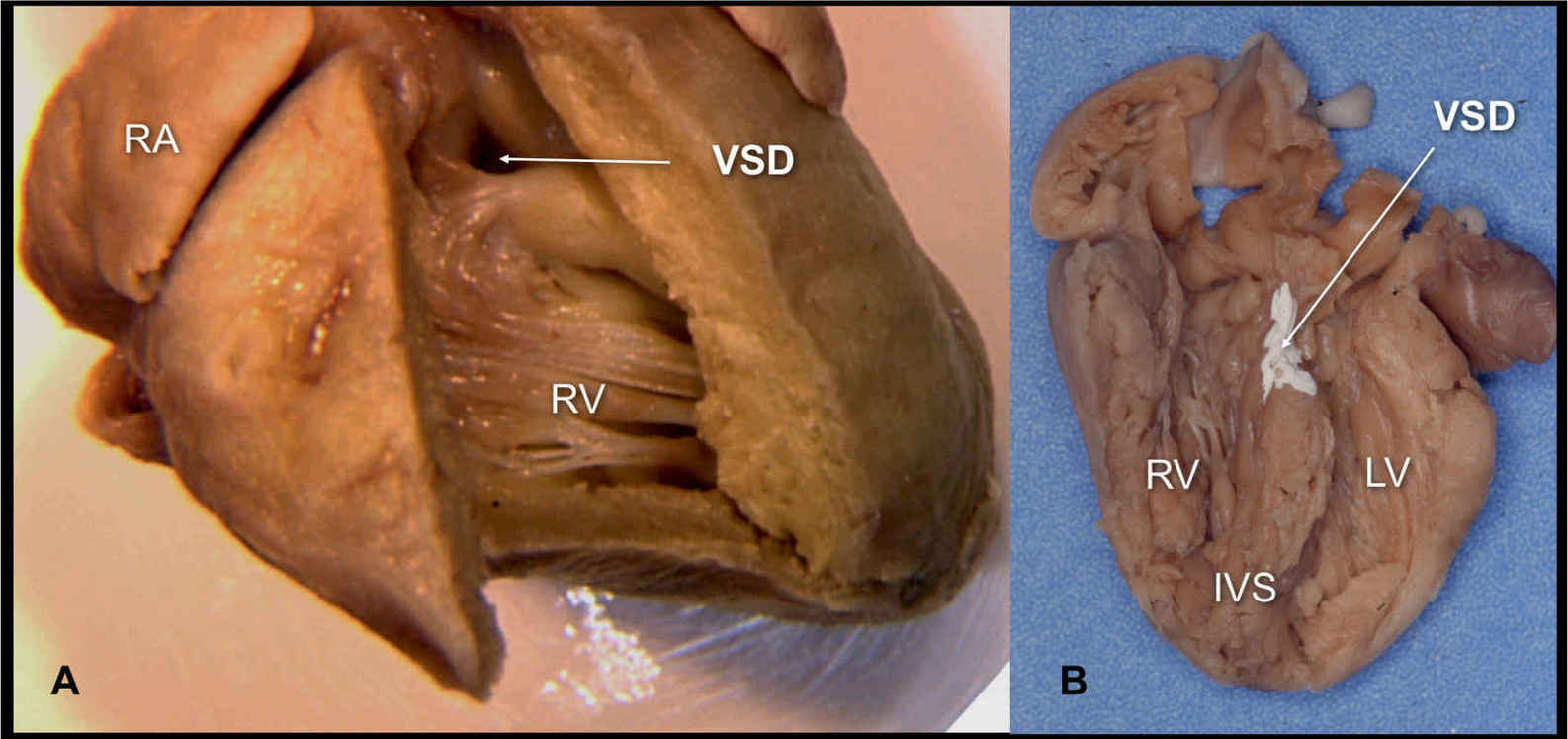
Figure 18.13: Two anatomic specimens of fetal hearts with ventricular septal defects (VSDs). A: The heart is opened from the right ventricle (RV) and a perimembranous VSD is seen (arrow). B: The heart is opened at the four-chamber view and a large inlet VSD is noted (arrow—the VSD is colored white for better demonstration). RA, right atrium; LV, left ventricle; IVS, interventricular septum.
Ultrasound Findings
Gray Scale
A VSD can be detected in the second and third trimesters of pregnancy with grayscale ultrasound when the size of the defect is more than 2 to 3 mm (Figs. 18.14 and 18.15). Smaller VSDs are often overseen on prenatal ultrasound and are occasionally detected by the routine use of color Doppler. The majority of reported VSDs in fetal series are detected when an extracardiac or cardiac anomaly is found and the heart is carefully examined, or when color Doppler is routinely used for cardiac screening.
Inlet VSDs are found in the region of the atrioventricular valves in the four-chamber plane (Figs. 18.14A and 18.16) and often are difficult to differentiate from a mild form of complete or partial AVSDs. Dropout artifacts on grayscale ultrasound and overlapping of color Doppler (bleeding artifact) can lead to false-positive diagnoses. The lateral or transverse view of the heart on grayscale ultrasound can be of help in reducing false-positive and false-negative diagnoses. The presence of linear insertion of the atrioventricular valves may be very suspicious for the presence of a mild form of an AVSD.
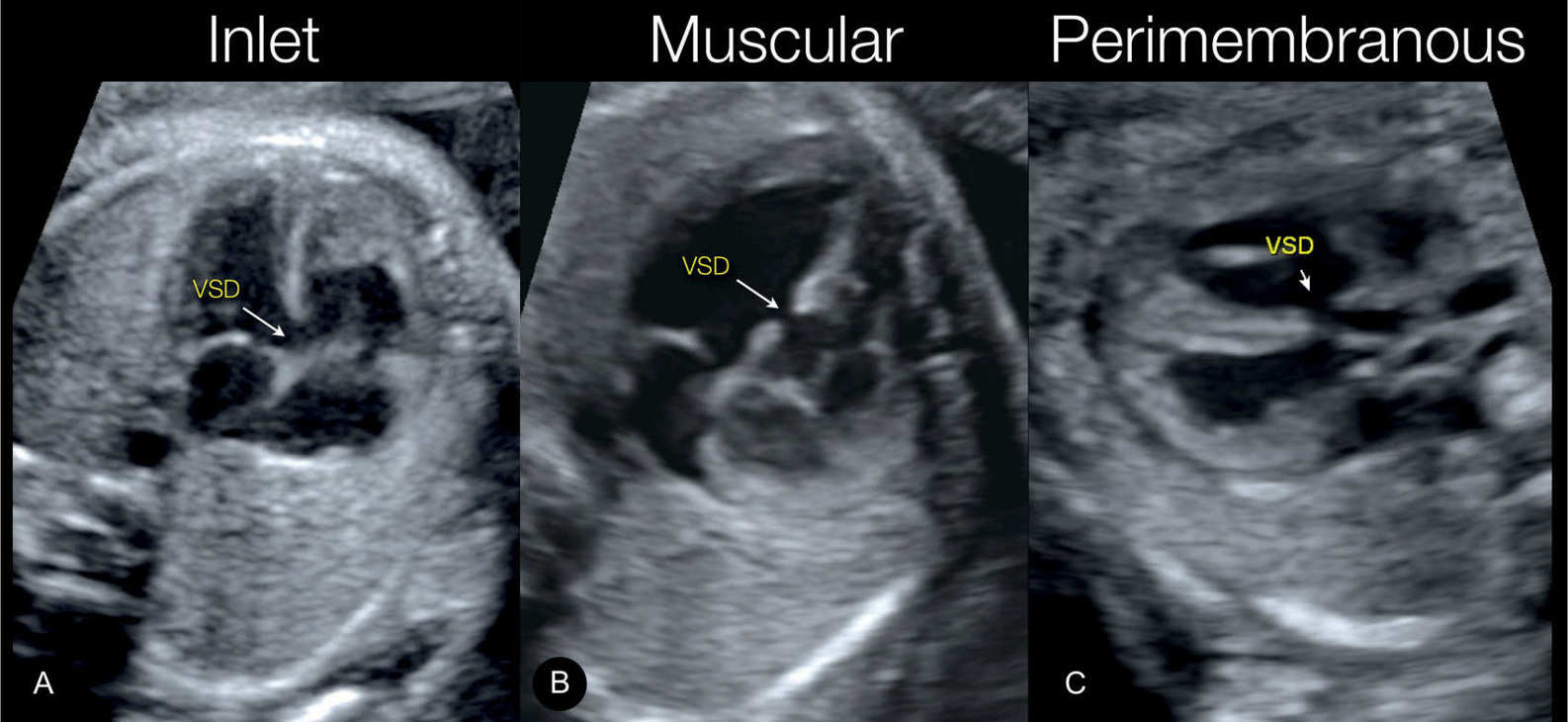
Figure 18.14: Three types of ventricular septal defects (VSDs) demonstrated on grayscale ultrasound. In A, an inlet VSD is shown in an apical four-chamber view plane. In B, a muscular VSD is shown in an apical four-chamber view plane, and in C, a perimembranous VSD is shown in a lateral five-chamber view plane.
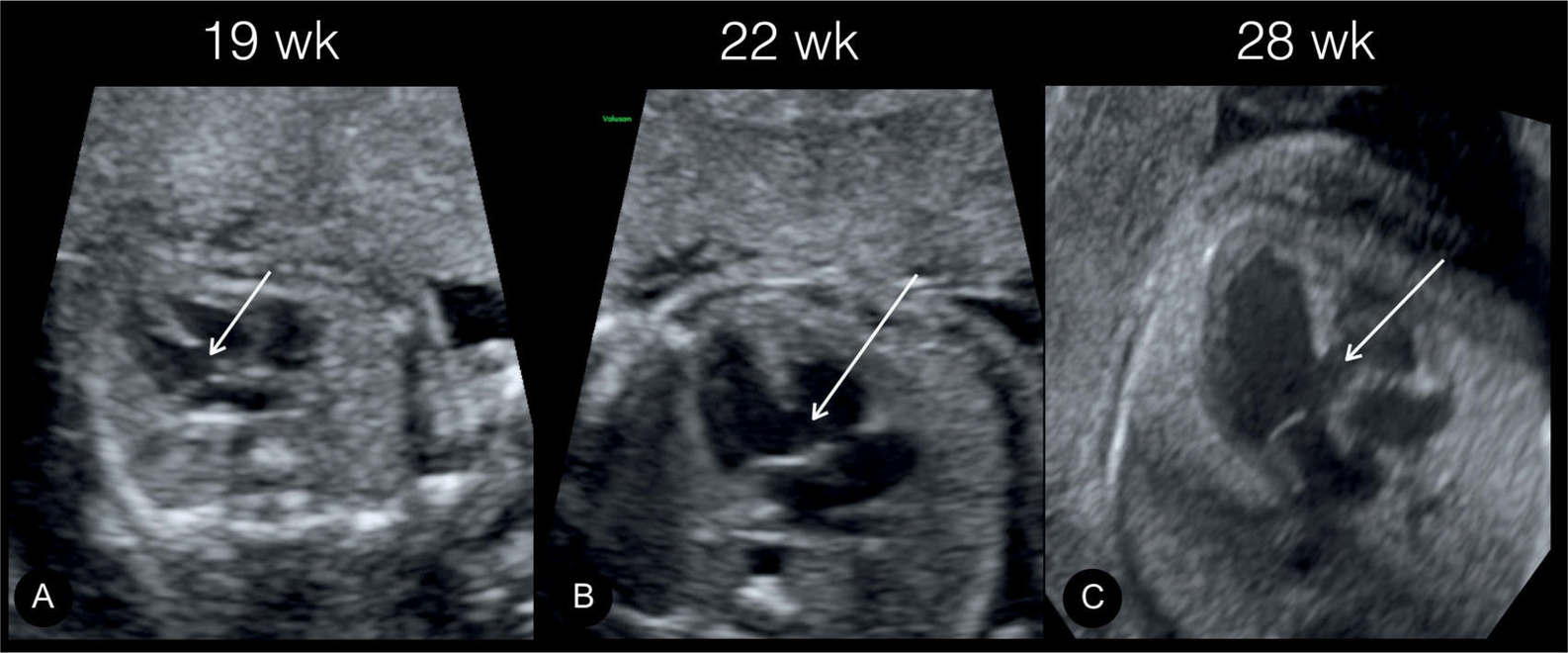
Figure 18.15: Apical four-chamber views (A–C) demonstrating an inlet ventricular septal defect (arrows) in the same fetus at 19 weeks in A, 22 weeks in B, and 28 weeks in C.
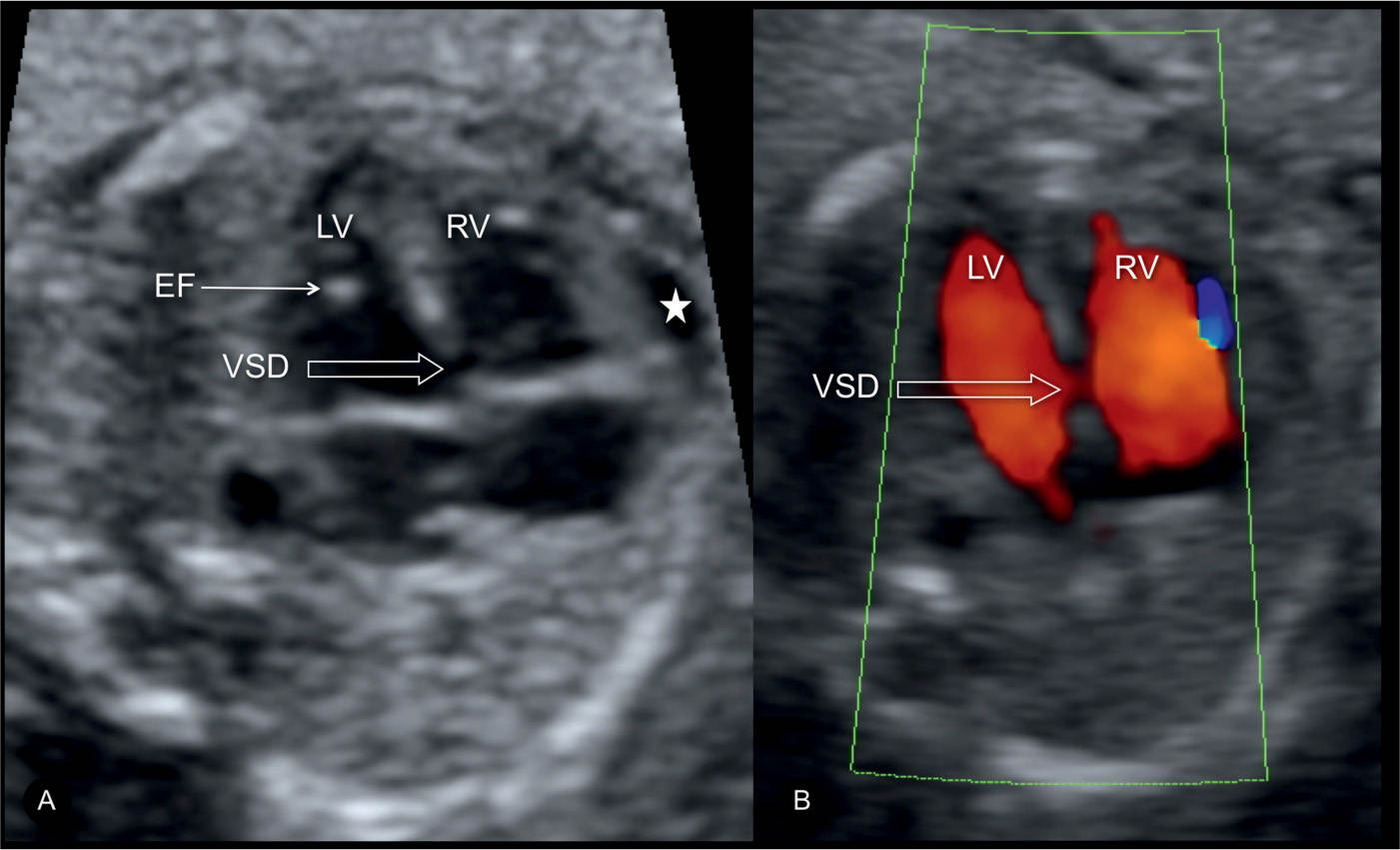
Figure 18.16: Inlet ventricular septal defect (VSD) demonstrated on gray scale (A, open arrow) and color Doppler (B, open arrow) at the four-chamber view. Echogenic intracardiac focus (EF) and pericardial effusion (star) are also shown in A. This fetus was found to have trisomy 21 on karyotypic analysis. LV, left ventricle; RV, right ventricle.
Muscular VSDs are rarely detected on grayscale ultrasound unless their size is large (>2–3 mm) (Figs. 18.14B, 18.17, and 18.18). They can be identified in the apical or transverse four-chamber views. In such cases, the borders of the VSD commonly appear echogenic (Figs. 18.17A and 18.18A), which can be an important hint in differentiating a true muscular VSD from artifact. The majority of muscular VSDs are detected by the routine use of color Doppler (Figs. 18.17 to 18.19). The shunt on color Doppler is typically bidirectional, and the most common location of VSDs is in the apical and midportion of the septum. At many centers, muscular VSDs are the most common VSD type detected prenatally (19).
The most common VSD detected on grayscale ultrasound is the perimembranous type, visualized in the five-chamber view. The first clue to its presence is in the interruption of the continuity between the ventricular septum and the medial wall of the ascending aorta (Figs. 18.20 and 18.21). When a perimembranous VSD is detected, detailed assessment of the great arteries is critical, given a strong association between perimembranous VSDs and conotruncal abnormalities (Table 18.2).
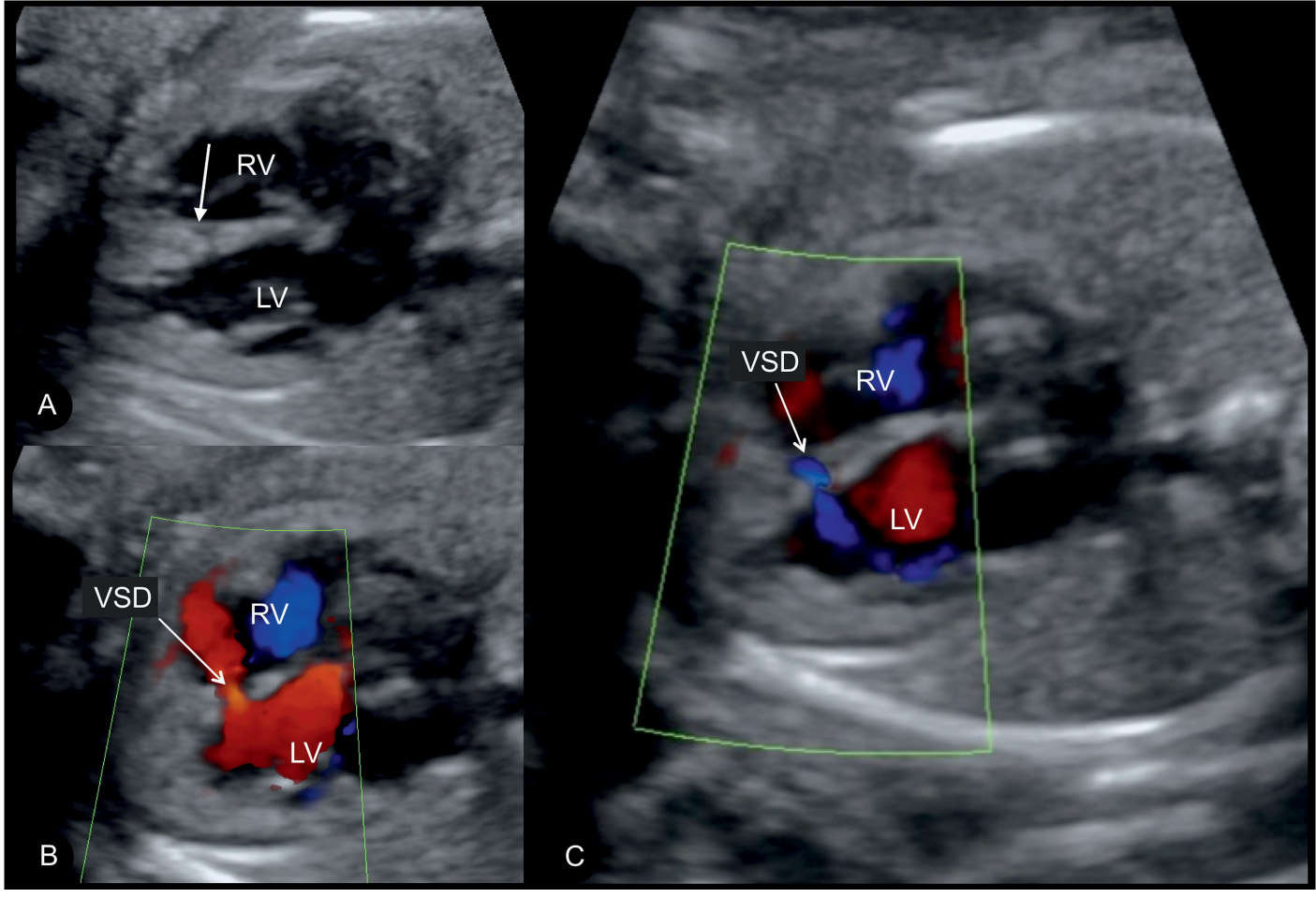
Figure 18.17: Axial four-chamber views in gray scale (A) and color Doppler (B and C) in a fetus with a small muscular ventricular septal defect (VSD) in the middle part of the interventricular septum. In A, the VSD is not clearly seen but suspected by the decrease in echogenicity of the septum (arrow). Color Doppler clearly demonstrates blood flow across the VSD in a bidirectional manner: from the left ventricle (LV) to the right ventricle (RV) in A and from the RV to the LV in B.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


