Vascular tumors
Vascular malformations
Simple
Combineda
of major named vessels
associated with other anomalies
Benign
Locally aggressive or borderline
Malignant
Capillary malformations
Lymphatic malformations
Venous malformations
Arteriovenous malformationsb
Arteriovenous fistulab
CVM, CLM
LVM, CLVM
CAVMb
CLAVMb
Others
See details
See list
The focus of this chapter will be on hemangiomas of infancy (HI), as epidemiologic and ethnic-specific data, albeit limited, are most available for this subgroup. The growth curves and typical clinical and molecular features of “typical hemangiomas” are depicted in Fig. 26.1 [3]. North and colleagues identified robust staining for the Glucose transporter 1 protein (GLUT1) in any growth stage of typical hemangiomas (and not expressed in other vascular anomalies), which is of diagnostic utility [4].
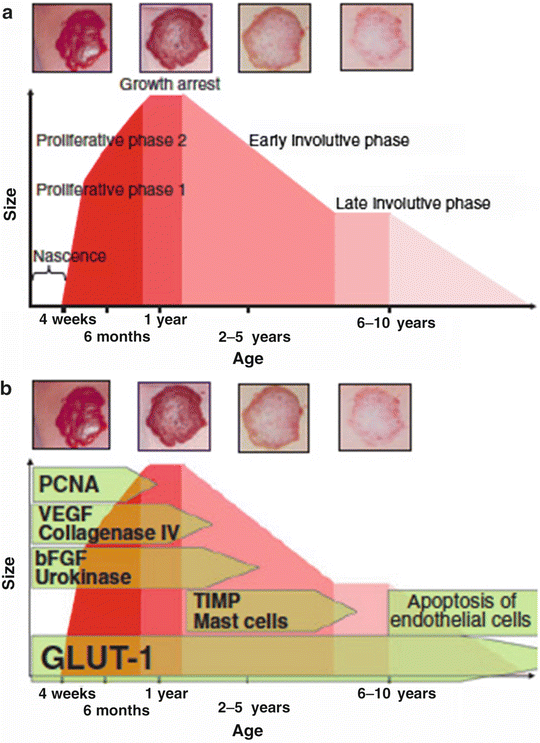

Fig. 26.1
Growth phases and properties of hemangioma of infancy. (a) Growth and regression of infantile hemangiomas (IH). (b) Molecular markers characterizing individual phases of IH. PCNA proliferating cell nuclear antigen, VEGF vascular endothelial growth factor, bFGF basic fibroblast growth factor, TIMP tissue inhibitor of metalloproteinases, GLUT-1 glucose transporter type1 ([3], #513)
Figure 26.2 depicts the time- and size-related variation in clinical presentation and behavior of the three types of hemangiomas (common hemangioma of infancy, rapidly involuting congenital hemangioma, and non-involuting congenital hemangioma). In-depth reviews comparing the various forms of hemangiomas are provided in recent reviews by Léauté-Labrèze, Hoeger, and Luu and colleagues [5–7].
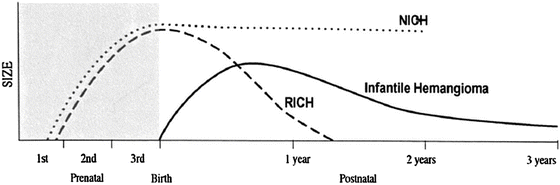

Fig. 26.2
Growth curves of hemangioma of infancy, rapidly involuting congenital hemangioma (RICH), and non-involuting congenital hemangioma (NICH)—note that only hemangiomas of infancy are GLUT1 positive ([8], #389)
Epidemiology
Few studies addressing racial/ethnic differences of HI
Predominance in Caucasian infants
Female gender, prematurity, and low birth weight are common risk factors
Prior to the 1980s, the word “hemangioma” was widely and loosely employed to encompass different vascular disorders. The classification proposed by Mulliken and Glowacki in 1982, and currently adopted with minor alterations, has changed considerably the nomenclature of vascular anomalies [1]. In addition, new categories for race/ethnicity have been created. As such, caution should be exerted in interpreting data originated from former publications. For instance, a large cohort study from 1983 groups salmon patches, stork bites, and Port wine stains under the heading of “capillary hemangioma,” with a resulting high incidence of hemangiomas [9].
The first study to estimate the occurrence of HI in different racial groups is credited to Pratt, in 1953 [10]. His patients were classified as either whites, negroes, or part negroes (sic), and “strawberry marks” were equally detected in 1 % of individuals of both groups. Very few reports approaching the occurrence of HI in different ethnic and racial groups have been published ever since (Table 26.2).
Table 26.2
Reported incidence of hemangiomas in various ethnic groups
Study | Year | Country | Number of patients | Race/ethnicitya | Nomenclature | Timing of examination | Incidence/prevalence |
|---|---|---|---|---|---|---|---|
Pratt [11] | 1953 | USA | 1,096 | Whites, Negroes, or part Negroes (sic) | Strawberry mark | First 8 days | 1 % for both groups |
Hidano et al. [12] | 1986 | Japan | 5,387 | NS | Strawberry mark | First 14 days | 1.7 % |
Rivers et al. [13] | 1990 | Australia | 420 | Caucasian, Mongolian, Australasian, unknown | None | First 7 days | None |
Kahana et al. [14] | 1995 | Israel | 1,672 | Jewish, Arab | Strawberry hemangioma | First 4 days | 1.3 % Jewish 1.48 % Arab |
Navas et al. [15] | Spain | 1,027 | NS | Other angiomas | First 14 days | 3.4 % | |
Boccardi et al. [16] | 2007 | Italy | 620 | European, Asian, North-African, South-American, other | None | First 3 days | None |
Shih et al. [17] | 20007 | Taiwan | 500 | NS | Hemangioma | First 2 days | 0.2 % |
Dickison et al. [18] | 2011 | Australia | 1,065 | Multipleb | Infantile hemangioma | First 6 weeks | 2.6 % |
Monteagudo et al. [19] | 2011 | Spain | 1,000 | White, Roma, Latin American, other | Hemangioma | First 3 days | 0.9 % |
Kanada et al. [20] | 2012 | USA | 594 | Caucasian, Hispanic, African-American, Asian, other | Infantile hemangioma | First 9 months | 6.2 % Hispanic 5.4 % Asian 4.5 % Caucasian 3.4 % African American 2.4 % other |
Hoornweg et al. [21] | 2012 | Netherlands | 2,204 | Caucasian, non-Caucasian | Infantile hemangioma | 2 year follow-up | 9.9 % |
Several country-specific studies report neonatal incidences of 0.17–1.3 % for “hemangiomas.” However, ascertainment in the immediate newborn period likely represents underreporting and/or erroneous diagnosis, as these studies lack longitudinal follow-up and diagnosis confirmation [22–24].
Due to the peculiar natural history of HI, discrepancies in prevalence/incidence rates in different studies are explained by distinct follow-up periods and timing of examination. Kilcline and Frieden, critically reviewed the literature and acknowledged methodological shortcomings in nomenclature and timing of these studies (i.e., studies in the newborn period may underestimate the true incidence of hemangiomas) [25]. However, these authors proposed that “the incidence is lower than the oft-cited 10 %, and likely closer to 4–5 %,” which was later corroborated by Kanada et al. who conducted a longitudinal study of nearly 600 infants in Southern California, with reported hemangiomas of infancy (HI, RICH, and NICH) in 4.9 % (4.5 % incidence of hemangiomas by 3 months of age), 45 % Caucasian, 25 % Hispanic, 4.7 % African-American, 9.4 % Asian, and 14.2 % “Other,” 12.1 % born preterm (<37 weeks gestational age), and 5.5 % twins [20]. These authors, who also recognized nevus simplex in 83 % and 0.3 % capillary malformation in their cohort, compared their results to those of several international studies. A longitudinal Australian study of >1,000 infants reported a 2.6 % incidence of infants who developed hemangiomas (confirmed by physicians) during the first 3–6 weeks of life, the majority female (5:1), of low birth weight (10 % <2,500 g), born at a gestational age <37 weeks, and conceived via in vitro fertilization (6.5 %)—although statistically this was not an independent risk factor. In this study, as in others, the majority of affected infants were Caucasian. Limitations include a low overall response rate to this questionnaire-based study (although the data was collected from >1,000 mothers), and the exclusion of very premature and very low birth weight infants [18]. Further, larger, and properly designed studies are needed to determine accurately the frequency of HI in different racial/ethnic groups.
It is postulated that HI predominate in fair-skinned patients and are relatively rare in dark-skinned ones. In the Netherlands, with a heavily predominant light-skinned population, prevalence rates of 9.9 % are reported, with 86 % of affected children being Caucasian [21]. In the USA, most likely the prototype country for multiracial population and ethnicity diversity, the occurrence of HI has been reported to be higher in white non-Hispanic [26]. A possible explanation is that the rise in preterm and low birth weight infants, two well-known risk factors for HI, has been more dramatic in the white non-Hispanic group [27]. A longitudinal Australian study of >1,000 infants reported a 2.6 % incidence of infants who developed hemangiomas (confirmed by physicians) during the first 3–6 weeks of life, the majority female (5:1), of low birth weight (10 % <2,500 g), born at a gestational age <37 weeks, and conceived via in vitro fertilization (6.5 %)—although statistically this was not an independent risk factor. In this study, as in others, the majority of affected infants were Caucasian. Limitations include a low overall response rate to this questionnaire-based study (although the data was collected from >1,000 mothers), and the exclusion of very premature and very low birth weight infants [18]. Females are markedly more affected than males, with ratios of up to 5:1. When only preterm infants are considered, the incidence of HI during the first year of life is 12.7 % [28]. Low birth weight (<2,500 g) is considered the most important risk factor for developing HI [27].
The Hemangioma Investigator Group, in a study of >1,000 infants, did not, however, provide an overall incidence of their cohort; they reported that hemangiomas of infancy were more likely seen in the following groups: female, white non-Hispanic (68.9 % vs. 14.4 % Hispanic, 2.8 % African-American), premature (20 % <37 week gestational age, 5.7 % <32 weeks gestational age), low birth weight (5.2 % <1,500 g, 13.3 % 1,500–2,499 g), multiple gestation, and born to mothers who had placental problems (placenta previa or preeclampsia) and of older maternal age. Family history of vascular lesions or hemangiomas in first-degree relatives was 32.9 % and 12.3 %, respectively [29]. Chen et al. conducted a study of 650 infants with hemangiomas followed in a Vascular Anomalies Center compared with 650 age-matched controls, and found a slightly increased incidence in females 2:1, twin gestation, <37-week gestational age, birth weight <2,500 g, in addition to maternal first trimester vaginal bleeding, progesterone therapy, and in vitro fertilization, with no substantial differences in older maternal age [30]. Li et al. reported a prospective study of infants in South China over a 3-year period and identified, in addition to multiple gestation, specific risk factors for hemangiomas in their cohort of >1,800 age-matched controls: lower level of maternal education, maternal manual labor, maternal use of medication in the periconceptional period, and family history of hemangiomas [31].
Clinical Features
Nearly all lesions visible at the end of the first month
Unique natural history with growth and involution phases
Neither gender nor ethnicity seems to affect the risk of complications
Course and Presentation
HI are the most common pediatric tumor. Usually absent at birth, or present as a precursor lesion (pink or ecchymotic macules, bruise-like coloring, telangiectatic patch with or without a halo of pallor, or ulceration), virtually all hemangiomas are detectable by the end of the first month of life. Their natural history is strikingly unique with a rapid early proliferative growth phase between 5.5 and 7.5 weeks of age, followed by a late slower growth period (age 6–9 months), and then by a long involuting phase that may last for many years [32]. It is estimated that hemangiomas reach 80 % of their final size by 3 months [33] and growth is completed at a median age of 3 years [34]. Small lesions usually involute with minimal or no scar. If left untreated, larger hemangiomas or those located in special areas such as lips, breast, and nose tip may leave unsightly scarring or even result in permanent disfigurement.
They may occur anywhere on the skin and mucous surfaces, but there is a clear preference for head, neck, and trunk. Based on their depth, HI can be classified as superficial, deep, and mixed (or combined). Superficial lesions, with proliferating vessels limited to the superficial dermis, are typically bright red and may be present as papule, nodule, or plaque, with a finely lobulated surface, hence the term “strawberry mark” (Fig. 26.3). Deep hemangiomas reach the deep dermis and subcutaneous tissue and present as nodule or tumor with a blue hue, often with a visible venous network. However, in some cases, the overlying skin may show no color changes (Fig. 26.4). Mixed hemangiomas share features of both superficial and deep subtypes (Fig. 26.5).
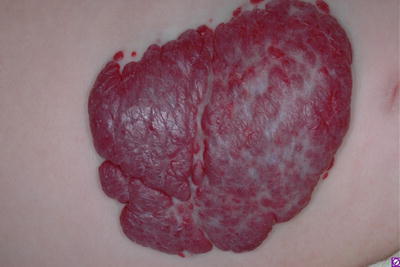
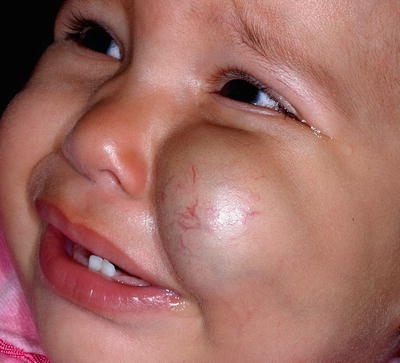
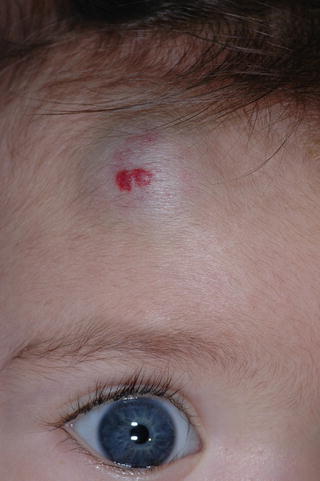

Fig. 26.3
Superficial hemangioma (“strawberry mark”)

Fig. 26.4
Deep hemangioma. Bluish tumor with overlying telangiectasias

Fig. 26.5
Mixed (compound) hemangioma. Note the bluish hue of the deep component contrasting with the bright red of the superficial portion
Morphologically, hemangiomas can be classified as localized, segmental, indeterminate, and multifocal. A localized hemangioma grows from a single focal point (Fig. 26.6). Isolated or grouped hemangiomas along recognizable developmental metameres or with dermatomal distribution are termed segmental (Fig. 26.7). Lesions that cannot be readily identified as either localized or segmental are classified as indeterminate. Multifocal hemangiomas are characterized by ≥8 noncontiguous cutaneous hemangiomas (Fig. 26.8). Hispanic patients are more prone to have segmental hemangiomas [35].
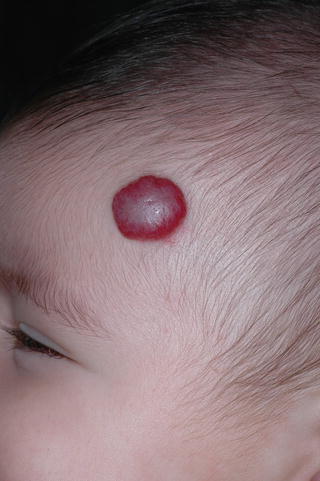
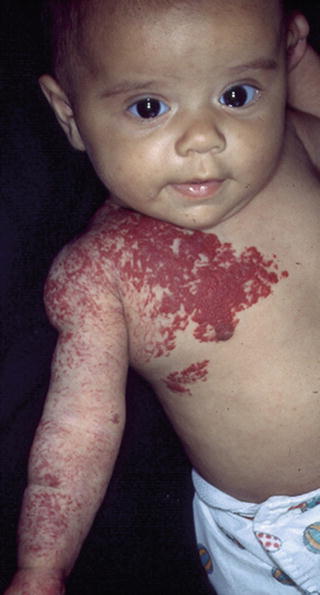
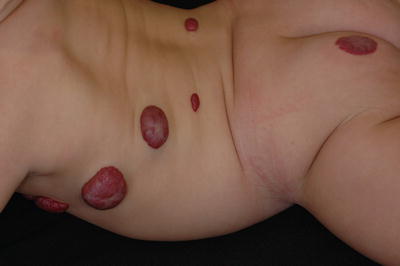

Fig. 26.6
Localized superficial hemangioma

Fig. 26.7
Segmental superficial hemangioma with dermatomal distribution

Fig. 26.8
Multifocal superficial hemangioma. This infant had no liver involvement, but presented with congenital agenesia of the left kidney and a cystic lesion in the right kidney
Although the vast majority of HI are represented by solitary lesions, more than one lesion can be detected in up to 30 % of affected infants, and approximately 3 % will develop ≥6 lesions [36]. The amount of cutaneous lesions correlates with the risk of visceral hemangiomas, most frequently hepatic.
Complications
Although only a minority of patients will experience complications of their HI along its natural history, physicians should to be aware of which circumstances favor the occurrence of such events. Neither sex nor ethnicity seems to play a role in increasing the risk for complications [29].
Ulceration
Ulceration is by far the most common complication and may affect as much as 23 % of patients with HI in a referral setting [37]. The pathogenesis of ulceration is still unclear but is thought to be the result of ischemia and necrosis stemming from trauma and friction, or of the rapid tumor growth exceeding its oxygenated blood supply. Large size and location (lips, diaper area, and neck) are associated with a greater risk of ulceration and propensity for ulceration is much higher in segmental than in localized hemangiomas (33.5 % versus 7.2 %) [36]. Resulting pain can be severe enough to cause sleeping disturbance and feeding difficulties (Fig. 26.9).
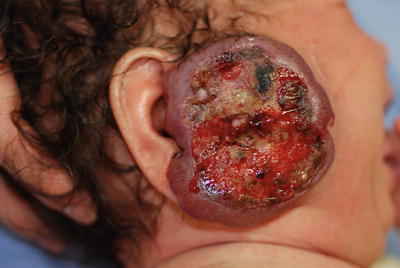

Fig. 26.9
Ulcerated hemangioma of the parotid area
Early white discoloration (average age of 2.6 months) of HI is highly predictive of impending ulceration [38]. This sign should be differentiated from the typically centrifugal discoloration that heralds spontaneous or treatment-induced involution of HI and usually begins after the completion of tumor growth (Fig. 26.10a, b).
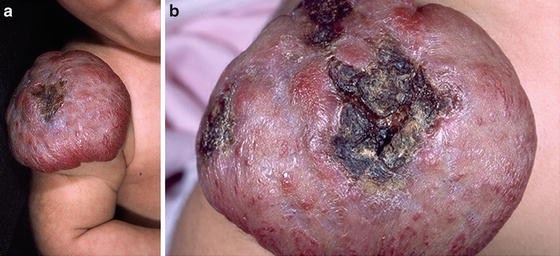

Fig. 26.10
Early discoloration sign. (a) Multiple focal whitening areas and imminent ulceration. (b) Ulcerations 9 days later
Visual Impairment
The most common complication of periocular hemangiomas is amblyopia (“lazy eye”), defined as a visual disturbance, without a detectable organic lesion of the eye, arising from inadequate stimulation of the visual cortex of the brain. Refractive errors (astigmatism, myopia), strabismus, or occlusion of the visual axis, by reducing or suppressing the proper image transmission to the brain, are the leading causes of amblyopia and can all be hemangioma induced [40]. The incidence of amblyopia in patients with periocular hemangioma has been reported to be as high as 73 %. However, a recent population-based study has estimated this rate to be 19 % [41].
Concerning the location, periocular hemangioma can be palpebral, extraconal, and/or intraconal. Extraocular muscles (superior, inferior, lateral, and medial rectus) appear in a cone-shaped arrangement in the retrobulbar space (Fig. 26.11). Lesions are considered extraconal or intraconal when located behind the bonny orbit, and outside or inside the muscle cone, respectively. There seems to be a strong association between extraconal and extraconal plus intraconal location and the risk of amblyopia and astigmatism. Palpebral lesions are also able to promote amblyopia and astigmatism in about 30 % of patients [42].
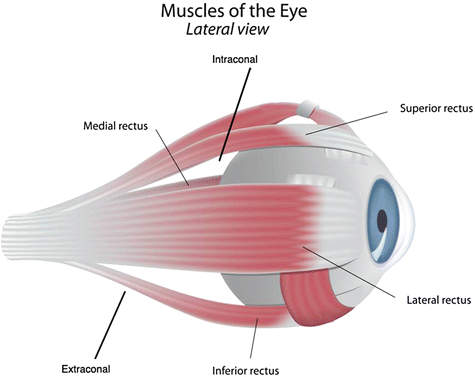

Fig. 26.11
Intraconal and extraconal locations of hemangiomas of the eye
Although small lesions, especially those on the lower eyelid, rarely induce visual dysfunctions, the potential to threaten or permanently compromise vision warrants close and regular ophthalmologic monitoring of virtually all HI of the orbit and eyelids. Imaging (MRI, CT, US) is mandatory to evaluate the tumor size and extension.
Airway Obstruction
Based on the systematic analysis of photographic data, Haggstrom et al. proposed four anatomic patterns (segments S1 to S4) for segmental facial hemangiomas. The frontotemporal S1 segment comprises the lateral forehead, anterior temporal scalp, and lateral frontal scalp. S2 and S3 segments correspond to the maxillary and mandibular prominences, respectively, while the S4 segment encompasses the medial frontal scalp, nasal bridge, nasal tip, ala, and philtrum [36].
The recognition that HI involving the so-called “beard area” (S3 segment-preauricular area, mandible, chin, lower lip, and anterior neck) is a marker for high risk of airway hemangioma is well established in the literature (Fig. 26.12). Bilateral presentation and involvement of multiple regions of the “beard area” increase this risk [43]. However, it should be kept in mind that airway hemangiomas have been demonstrated in association with extrafacial HI [44], with facial HI outside the S3 segment [45], and even in the absence of cutaneous HI.
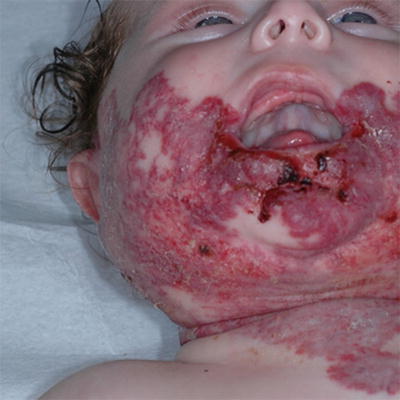

Fig. 26.12
Hemangioma of the “beard area.” This infant had no airway hemangioma
Hoarse cry, biphasic (inspiration and expiration) stridor, and noisy breathing are classic signs of subglottic hemangiomas. Since many of these lesions are, in practice, accidentally diagnosed during bronchoscopy, respiratory symptoms should prompt otolaryngologic evaluation regardless of the presence of cutaneous HI [45, 46].
Disfigurement
The dogma of the non-interventional approach to HI, which reigned over many decades in the past, was undoubtedly responsible for numerous cases of severe and permanent disfigurement coupled with an enormous impact in these patients’ quality of life. Knowledge gathered in recent years, together with the availability of novel therapeutic modalities, allows for the proper management of HI with reduced, or even suppressed, unpleasant aesthetic results. Along with ulceration, prevention of disfigurement is the most common reason for active treatment.
Some anatomic areas are more prone to disfigurement. Ulceration on the lip may result in permanent distortion and lesions on the nasal tip (Cyrano’s nose) may cause significant aesthetic impairment (Fig. 26.13). Even small-sized HI, if sessile or pedunculated, can heal with significant fibrofatty residuum (Fig. 26.14a–c). The breast area in girls is another site of concern.
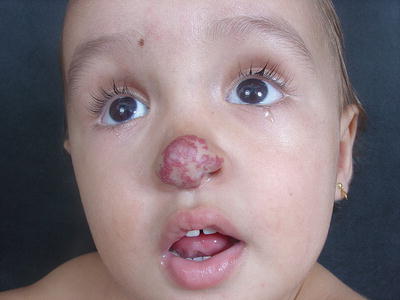
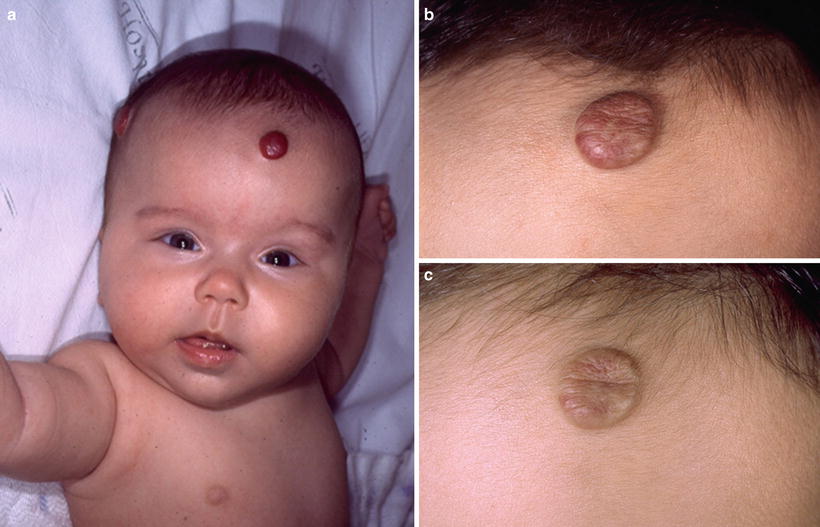

Fig. 26.13
Cyrano’s nose: bulging of the tip of nose

Fig. 26.14
Spontaneous resolution of hemangioma. (a) At age 3 months. (b) Flattening and discoloration at age 2 years. (c) Fibrofatty residuum at age 3 years
Other Complications
Huang et al. reported a case of severe hypothyroidism in a 3-month-old infant with massive hepatic hemangiomas and high levels of type 3 iodothyronine deiodinase (D3) activity in the hemangioma tissue. This enzyme catalyzes the conversion of thyroxine to reverse triiodothyronine as well as the conversion of triiodothyronine to 3,3′-diiodothyronine, both of which are biologically inactive [47]. The authors postulated that the degradation of the thyroid hormone generated by the intense enzymatic activity of the tumor exceeds the infant’s gland capacity to synthesize it.
Subsequently, few cases of HI-induced hypothyroidism have been published. Characteristically, these are associated with large visceral hemangiomas (liver, parotid) [48].
High output cardiac failure is a life-threatening complication generally related to high flow tumors such as hepatic hemangiomas. Approximately 16 % of infants with ≥5 cutaneous hemangiomas have hemangioma of the liver and, hence, routine abdominal US should be performed under these circumstances [49] (Fig. 26.15a–c).
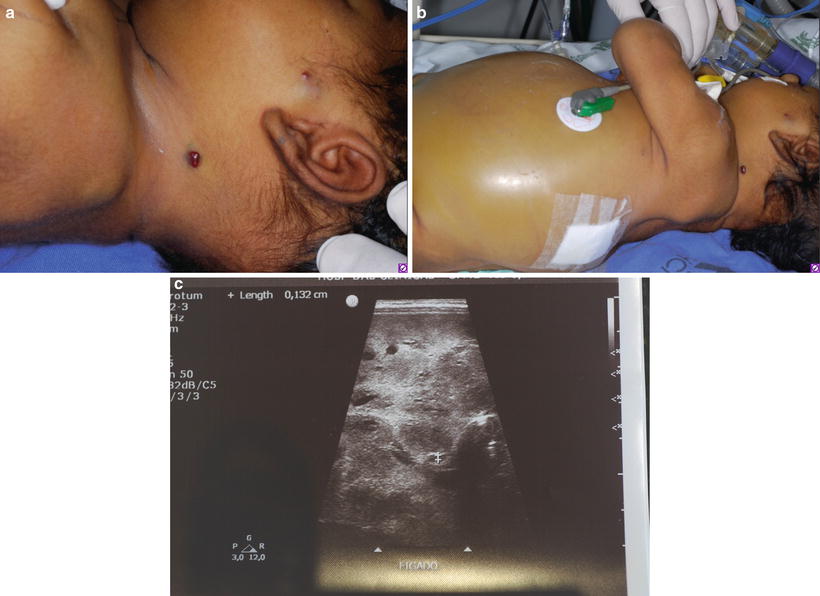

Fig. 26.15
Multifocal hemangioma with fatal outcome. This patient had 11 small-sized GLUT1 positive lesions. (a) Hemangiomas on the face, neck, and earlobe. (b) Abdominal distension secondary to massive liver invasion. (c) US showing diffuse liver hemangiomas
Obstruction of the external auditory canal may lead to otitis and decreased auditory conduction with speech delay.
Associated Structural Abnormalities
The acronym PHACE(s) (posterior fossa malformations, hemangiomas, arterial anomalies, coarctation and other cardiac defects, eye abnormalities, and sternal cleft) describes the association of HI and other anomalies with diagnostic criteria established by consensus [50]. Up to 30 % of patients with large (>5 cm), facial segmental hemangiomas will present PHACE(s) syndrome. Although the risk is higher for lesions located along frontotemporal (S1) or mandibular (S3) areas, any segment may be involved [51]. Hispanic infants are more likely to have their segmental hemangiomas associated with PHACE(s) syndrome [35]. On the other hand, hemangioma distribution does not predict the occurrence of cardiovascular anomalies [52].
LUMBAR (lower body hemangioma and other cutaneous defects, urogenital anomalies, ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies, and renal anomalies), PELVIS (perineal hemangioma, external genitalia malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus, and skin tag syndrome), and SACRAL (spinal dysraphism, anogenital, cutaneous, renal, and urological anomalies, associated with an angioma of lumbosacral localization) are acronyms that refer to the association of large segmental HI of the lumbosacral or perineal areas and underlying structural abnormalities.
Treatment
Timely referral is essential, as early treatment can prevent unwanted morbidities.
Treatment options may vary based on timing of treatment onset, location, and type of hemangioma
Combined therapies may be preferable.
Considering typical hemangiomas of infancy, timely referral is indispensable to determine whether further evaluation or treatment is warranted. Age of the patient, hemangioma location, its morphology, size, the number of lesions, associated (or impending) symptoms, and/or structural anomalies contribute to management decisions. Proactive early therapy can prevent the need for more invasive interventions and/or surgery in the future.
Laser Treatment
Early pulsed dye laser may be beneficial in preventing proliferation in early superficial hemangiomas, whereas pulsed dye or other lasers may ameliorate residual telangiectasias or contour irregularities after involution [53–56]. Regarding laser treatment, skin of color is pertinent to the treatment of vascular anomalies. Chaplin and Jablonski note that “melanin accounts for most of the variation in the visual appearance of human skin,” demonstrating that skin color correlates with environmental UV radiation, and variations in human melanin pigmentation are adaptive to ameliorate the consequences of UV radiation (damage to dermal blood vessels, sweat glands, etc.). They further posit “skin reflectance, particularly in the wavelength approximating the absorption maximum of oxyhemoglobin (545 nm), has been optimized by natural selection to balance the conflicting requirements of prevention of folate photolysis (photoprotection) and previtamin D3 synthesis (requiring UV penetration)” [57, 58]. Dark (melanized) skin (as seen in equatorial and tropical climates) provides photoprotection and abrogates UV-damage-related interference with thermoregulation. Conversely, fair-skinned individuals (in the northernmost locations) require UV exposure for D3 synthesis. These findings are anthropologically interesting, and relevant to the current laser therapies of vascular lesions.
Various laser treatments for vascular anomalies (pulsed dye, Q-switched ruby, Q-switched neodymium:yttrium-aluminum-garnet (Nd:YAG), Q-switched alexandrite, intense pulsed light, intralesional photocoagulation (ILP), fractional CO2, and potassium-titanyl-phosphate (KTP) 1,064-nm lasers) have been used, tailored to the type and location of vascular lesion, age of the patient, and desired outcome [54, 59–62]. Pulsed dye laser therapy, which is typically used for hemangiomas and capillary malformations, is delivered at a wavelength of 595 nm in the green-yellow spectrum and is customary for cutaneous vascular lesions (hemangiomas, telangiectasias, capillary malformations) (Fig. 26.16).