Background
Preterm prelabor rupture of membranes is frequently complicated/accompanied by infection and inflammation in the amniotic cavity. A point-of-care determination of amniotic fluid interleukin-6 has been shown to be a potentially clinically useful approach to assess inflammatory status of the amniotic cavity. Amniocentesis in preterm prelabor rupture of membranes is not broadly used in clinical practice, and therefore, a shift toward a noninvasive amniotic fluid sampling method is needed.
Objective
The first aim of this study was to evaluate the association between the point-of-care vaginal and amniotic fluid interleukin-6 concentrations in fresh unprocessed samples obtained simultaneously. The second goal was to determine the diagnostic indices and predictive value of the point-of-care assessment of vaginal fluid interleukin-6 concentration in the identification of microbial invasion of the amniotic cavity, intraamniotic inflammation, and microbial-associated intraamniotic inflammation in patients with preterm prelabor rupture of membranes.
Study Design
A prospective cohort study was conducted in women with singleton gestation complicated by preterm prelabor rupture of membranes at between 24+0 and 36+6 weeks. A total of 153 women with singleton pregnancies were included in this study. Vaginal fluid was obtained from the posterior vaginal fornix by aspiration with a sterile urine sample tube with a suction tip. Amniotic fluid was obtained by transabdominal amniocentesis. Interleukin-6 concentrations were assessed with a lateral flow immunoassay in both fluids immediately after sampling. Microbial invasion of the amniotic cavity was determined based on a positive polymerase chain reaction analysis. Intraamniotic inflammation was defined as an amniotic fluid point-of-care interleukin-6 concentration ≥745 pg/mL.
Results
Several results were obtained in this study. First, it was possible to perform the point-of-care assessment of interleukin-6 in vaginal fluid in 92% of the women (141 of 153), and only those women were included in the analyses. Second, the rate of microbial invasion of the amniotic cavity and intraamniotic inflammation was 26% (36 of 141) and 19% (27 of 141), respectively. Microbial-associated intraamniotic inflammation was identified in 12% of the women (17 of 141). Third, a strong positive correlation was found between the interleukin-6 concentrations in vaginal and amniotic fluids (Spearman rho 0.68; P < .0001). Fourth, the presence of microbial invasion of the amniotic cavity, intraamniotic inflammation, or microbial-associated intraamniotic inflammation was associated with higher vaginal fluid interleukin-6 concentrations in both crude and adjusted analyses. Fifth, a vaginal fluid interleukin-6 concentration of 2500 pg/mL was determined to be the best cutoff value for the identification of microbial invasion of the amniotic cavity (sensitivity of 53% [19 of 36], specificity of 89% [93 of 104], positive predictive value of 63% [19 of 30], negative predictive value of 85% [93 of 110], positive likelihood ratio of 5.0 [95% confidence interval, 2.5–9.5], and negative likelihood ratio of 0.5 [95% confidence interval, 0.4–0.8]); intraamniotic inflammation (sensitivity of 74% [20/27], specificity of 91% [104/114], positive predictive value of 67% [20 of 30], negative predictive value of 94% [104 of 111], positive likelihood ratio of 8.4 [95% confidence interval, 4.5–15.9], and negative likelihood ratio of 0.3 [95% confidence interval, 0.2–0.5]); and microbial-associated intraamniotic inflammation (sensitivity of 100% [17 of 17], specificity of 90% [111 of 124), positive predictive value of 57% [17 of 30], negative predictive value of 100% [111 of 111], positive likelihood ratio of 9.5 [95% confidence interval, 5.7–16.0], and negative likelihood ratio of 0).
Conclusion
The point-of-care assessment of interleukin-6 in vaginal fluid is an easy, rapid, noninvasive, and inexpensive method for the identification of intraamniotic inflammation and microbial-associated intraamniotic inflammation in preterm prelabor rupture of membranes pregnancies, showing good specificity and negative predictive value.
Preterm prelabor rupture of membranes, characterized as rupture of the fetal membranes with leakage of amniotic fluid before onset of regular uterine activity prior to gestational age 37 weeks, complicates about one third of all preterm deliveries and approximately 3% of all deliveries. Preterm prelabor rupture of membranes is frequently complicated by specific infection-related and inflammatory conditions such as microbial invasion of the amniotic cavity, intraamniotic inflammation (either microbial associated or sterile), and acute histological chorioamnionitis. The presence of these complications is associated with adverse pregnancy and neonatal outcomes.
Multiple protein mediators as evaluated by various analytical techniques (enzyme-linked immunosorbent assay, multiplex, proteomic) have been suggested as potential markers for the identification of preterm prelabor rupture of membrane complications. Among them, the pleotropic cytokine interleukin-6 has been shown to be an efficient marker of microbial invasion of the amniotic cavity and intraamniotic inflammation that is not inferior to modern proteomic markers. Recent studies have reported that the point-of-care assessment of amniotic fluid interleukin-6 is a clinically relevant approach for the early determination of the infection related and inflammatory status of the amniotic cavity.
Currently the early identification of preterm prelabor rupture of membranes complications is mainly based on the evaluation of amniotic fluid obtained by transabdominal amniocentesis . This invasive procedure can be especially demanding in scenarios with a low amount of residual amniotic fluid. For this reason, universal use of transabdominal amniocentesis in preterm prelabor rupture of membranes cannot be anticipated, regardless of the quality of the ultrasound machine or the experience of the operator. On the other hand, a recent study has suggested amniotic fluid can be collected noninvasively in women with preterm prelabor rupture of membranes with the use of a transcervical collector.
Interleukin-6 assessment has also been found to be effective in cervical and vaginal fluid. There are reports that point-of-care interleukin-6 assessment in cervical and vaginal fluids performs well but needs to be developed to have functionality in the point-of-care setting.
Various approaches have been suggested for cervical and vaginal fluid sampling: a Dacron swab, a sponge, a syringe, plastic pipettes, and the use of a garlic press to squeeze vaginal secretions out of a sanitary pad. However, from the clinical point of view, it is important to have an easy and feasible sampling procedure followed by a rapid evaluation of the fresh sample, ideally directly in the labor room.
The first aim of this study was to evaluate the association between interleukin-6 concentrations in fresh unprocessed vaginal fluid and amniotic fluid. The second goal was to determine the diagnostic indices and predictive value of point-of-care vaginal fluid interleukin-6 assessment in the identification of microbial invasion of the amniotic cavity, intraamniotic inflammation, and microbial-associated intraamniotic inflammation in patients with preterm prelabor rupture of membranes.
Materials and Methods
The patient population
Between August 2014 and March 2016, a prospective cohort study was conducted in pregnant women at gestational ages between 24+0 and 36+6 weeks who were admitted to the Department of Obstetrics and Gynecology, University Hospital Hradec Kralove, Czech Republic.
Women with maternal age ≥18 years having a singleton pregnancy complicated by preterm prelabor rupture of membranes were invited to participate in the study. Women were excluded if they had pregestational or gestational diabetes, gestational hypertension, preeclampsia, significant vaginal bleeding, or their fetus had signs of fetal growth restriction, fetal hypoxia, congenital abnormality, or chromosomal abnormality.
Gestational age was established by first-trimester fetal biometry. Women with preterm prelabor rupture of membranes at less than 34 weeks of gestation were treated with corticosteroids, tocolytics for 48 hours, and antibiotics, whereas no treatment except antibiotics was used after 34 weeks. Women with a proven microbial-associated intraamniotic inflammation beyond 28 gestational weeks were managed actively. Women managed actively did not receive tocolytics. They were treated only with corticosteroids and antibiotics, and labor was induced or an elective cesarean delivery was performed after finalizing corticosteroid treatment but no later than 72 hours after the rupture of the membranes. The remaining women were managed conservatively.
Preterm prelabor rupture of membranes was diagnosed by examination with a sterile speculum to verify the pooling of amniotic fluid in the vagina, and when necessary, it was confirmed only by the presence of insulin-like growth factor binding proteins (ACTIM prelabor rupture of membranes test; MedixBiochemica, Kauniainen, Finland) in the vaginal fluid.
This study was approved by the institutional review board committee (July, 2014; number 201407 S14P), and informed consent was received from all participants.
Biological samples and analysis
Vaginal and amniotic fluid samples were collected simultaneously at the time of admission prior to the administration of corticosteroids, antibiotics, or tocolytics. Vaginal fluid was obtained from the posterior fornix during the examination in a sterile speculum. A total of 100 μL of noncentrifuged vaginal fluid was used for the point-of-care assessment of interleukin-6 concentration.
Ultrasound-guided transabdominal amniocentesis was performed, and approximately 3 mL of amniotic fluid was aspirated. A total of 100 μL of noncentrifuged amniotic fluid was used for the point-of-care assessment of interleukin-6 concentration.
The remaining amniotic fluid was immediately divided into 2 polypropylene tubes. The first tube, containing a noncentrifuged sample, was immediately transported to the microbiology laboratory for polymerase chain reaction testing for Ureaplasma species, Mycoplasma hominis , and Chlamydia trachomatis and to evaluate 16S ribosomal RNA. The second tube was centrifuged for 15 minutes at 2000 × g to remove cells and debris, divided into aliquots, and stored at –70°C.
Vaginal fluid collection
Vaginal fluid was aspirated from the posterior fornix of the vagina with a Urine Monovette 10 mL (Sarstedt AG & Co, Nümbrecht, Germany) during a speculum examination. The Urine Monovette is a sterile urine sample tube that includes a suction tip for drawing urine from the collection container ( Figure 1 ).
Amniotic and vaginal fluid interleukin-6 concentration
The interleukin-6 concentration in fresh noncentrifuged amniotic and vaginal fluids was assessed with a Millenia QuickLine interleukin-6 lateral flow immunoassay using the Milenia POCScan Reader (Millenia Biotec GmbH, Giessen, Germany). The measurement range was 50,000–10,000 pg/mL. The intraassay and interassay coefficients of variation were 12.1% and 15.5%, respectively. The equipment was housed in the labor and delivery ward, and the tests were also performed in that location.
Detection of Ureaplasma species, Mycoplasma hominis , and Chlamydia trachomatis
DNA was isolated from the amniotic fluid with a QIAamp DNA minikit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions (using the protocol for the isolation of bacterial DNA from biological fluids). Real-time polymerase chain reaction was performed on a Rotor-Gene 6000 instrument (QIAGEN) with the commercial kit AmpliSens Chlamydia trachomatis/Ureaplasma/Mycoplasma hominis -FRT (Federal State Institution of Science, Central Research Institute of Epidemiology, Moscow, Russia) to detect the DNA from Ureaplasma species, Mycoplasma hominis , and Chlamydia trachomatis in a common polymerase chain reaction tube.
As a control, we included polymerase chain reaction run for beta-actin , a housekeeping gene, to assess the presence of polymerase chain reaction inhibitors. The amount of Ureaplasma species DNA in copies per milliliter was determined by an absolute quantification technique using an external calibration curve. Plasmid DNA (pCR4; Invitrogen, Carlsbad, CA) was used to prepare the calibration curve.
Detection of other bacteria in amniotic fluid
Bacterial DNA was identified by polymerase chain reaction targeting the 16S ribosomal RNA gene with the following primers: 5′-CCAGACTCCTACGGGAGGCAG-3′ (V3 region), 5′-ACATTTCACAACACGAGCTGACGA-3′ (V6 region).
Each individual reaction contained 3 μL of target DNA, 500 nM of forward and reverse primers, and Q5 high-fidelity DNA polymerase (New England Biolabs, Beverly, MA) in a total volume of 25 μL. The amplification was performed in a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA).
The products were visualized on an agarose gel. Positive reactions yielded products of 950 bp, which were subsequently analyzed by sequencing. The 16S ribosomal RNA polymerase chain reaction products were cleaned and used in sequencing polymerase chain reactions using the previously mentioned primers and the BigDye Terminator kit, version 3.1 (Thermo Fisher Scientific, Waltham, MA). Bacteria were then genotyped using the sequences obtained in BLAST and SepsiTest BLAST.
Diagnosis of microbial invasion of the amniotic cavity
Microbial invasion of the amniotic cavity was determined based on a positive polymerase chain reaction analysis for Ureaplasma species, Mycoplasma hominis , or Chlamydia trachomatis or by positivity for the 16S ribosomal RNA gene. Specific polymerase chain reaction for Ureaplasma species, Mycoplasma hominis , or Chlamydia trachomatis has a sensitivity of 50–100 copies/mL. The sensitivity of our method for detection of bacterial 16S ribosomal RNA, verified by DNA of Streptococcus agalactiae , Streptococcus mutans , and Haemophilus influenza, is 150–700 copies/mL. Sanger sequencing of 16S ribosomal RNA is possible only when the concentration of bacterial DNA in amniotic fluid is at least 1500–7000 copies/mL.
Definition of intraamniotic inflammation
Intraamniotic inflammation in preterm prelabor rupture of membrane pregnancies was defined as an amniotic fluid point-of-care interleukin-6 concentration ≥745 pg/mL.
Definition of microbial-associated intraamniotic inflammation
Microbial-associated intraamniotic inflammation was defined as the presence of microbial invasion of the amniotic cavity along with an amniotic fluid point-of-care interleukin-6 concentration ≥745 pg/mL.
Statistical analyses
The demographic characteristics were compared by the nonparametric Mann-Whitney U test and the Kruskal-Wallis test for continuous variables and presented as median values (range). Categorical variables were compared with the χ 2 test and presented as numbers (percentages). The normality of the data was tested using the D’Agostino-Pearson omnibus normality test and the Shapiro-Wilk test.
Because the amniotic and vaginal fluid concentrations of interleukin-6 were not normally distributed, nonparametric tests (Mann-Whitney U test and Kruskal-Wallis) were used for the analyses, and data were presented as median values (interquartile range). Correlation between the interleukin-6 concentrations in the vaginal fluid and amniotic fluid samples was assessed using Spearman’s correlation coefficients. The Spearman partial correlation was performed to adjust for gestational age at sampling.
Receiver-operator characteristic curves were constructed to determine the predictive value of interleukin-6 in amniotic fluid for the presence of microbial invasion of the amniotic cavity and in vaginal fluid for the presence of microbial invasion of the amniotic cavity, intraamniotic inflammation, and microbial-associated intraamniotic inflammation. The areas under receiver-operator characteristic curves to determine the predictive value of interleukin-6 in amniotic and vaginal fluid for the presence of microbial invasion of the amniotic cavity was compared by using the methods of DeLong et al.
Differences were considered statistically significant at P < .05. All P values were obtained from 2-sided tests, and all statistical analyses were performed using GraphPad Prism 6 for Mac OS X (GraphPad Software, San Diego, CA), the SPSS 19.0 statistical package for Mac OS X (SPSS Inc, Chicago, IL), and MedCalc Statistical Software version 12.7.7 (MedCalc Software bvba, Ostend, Belgium).
Results
Clinical characteristics of the population
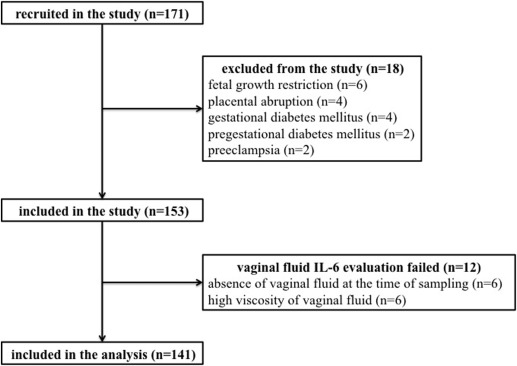
In total, 171 women with preterm prelabor rupture of membranes at gestational ages between 24+0 and 36+6 weeks were recruited. Eighteen women were excluded from the study owing to fetal growth restriction (n = 6), placental abruption with bleeding (n = 4), gestational diabetes mellitus (n = 4), pregestational diabetes mellitus (n = 2), and preeclampsia (n = 2). The remaining 153 women were included in the study.
It was possible to perform point-of-care assessment of interleukin-6 in vaginal fluid in 92% of the women (141 of 153), and only those women were included in the analysis. In the remaining 12 women, the vaginal fluid interleukin-6 evaluation failed due to an absence of vaginal fluid in the posterior fornix at the time of sampling (n = 5) or high viscosity of the vaginal fluid that made point-of-care interleukin-6 evaluation impossible (n = 7).
The flow diagram describing the selection of women is demonstrated in Figure 2 . The demographic and clinical data of the women according to the presence and absence of microbial invasion of the amniotic cavity, intraamniotic inflammation, and microbial-associated intraamniotic inflammation are shown in Table 1 .
| With MIAC (n = 36) | Without MIAC (n = 105) | P value a | With intraamniotic inflammation (n = 27) | Without intraamniotic inflammation (n = 114) | P value b | With microbial-associated intraamniotic inflammation (n = 17) | Without microbial-associated intraamniotic inflammation (n = 124) | P value c | |
|---|---|---|---|---|---|---|---|---|---|
| Maternal age, y | 31.1 ± 5.4 | 31.7 ± 4.9 | .56 | 31.4 ± 4.5 | 31.6 ± 5.1 | .76 | 31.7 ± 4.8 | 31.5 ± 5.1 | .88 |
| Primiparous | 18 (50%) | 47 (45%) | .59 | 15 (55%) | 50 (44%) | .27 | 8 (47%) | 57 (46%) | .93 |
| Prepregnancy body mass index, kg/m 2 | 24.2 (18.1–36.2) | 23.6 (17.5–39.4) | .79 | 24.3 (18.1–36.2) | 23.7 (17.5–39.4) | .97 | 24.3 (18.1–36.2) | 23.7 (17.5–39.4) | .85 |
| Smoking | 8 (22%) | 9 (9%) | .03 d | 7 (26%) | 10 (9%) | .01 d | 5 (29%) | 12 (10%) | .02 d |
| Gestational age at admission, week plus days | 32+4 (24+0–36+2) | 33+6 (24+0–36+5) | .04 d | 30+0 (24+0–36+0) | 34+0 (24+0–36+5) | < .0001 d | 30+0 (24+0–36+0) | 33+6 (24+0–36+5) | < .0001 d |
| Gestational age at delivery, week plus days | 33+0 (25+0–36+2) | 34+2 (24+0–37+0) | .02 d | 30+4 (25+0–36+0) | 34+2 (24+0–37+0) | < .0001 d | 30+1 (25+0–36+0) | 34+1 (24+0–37+0) | < .0001 d |
| Delivery before gestational age 28 wks | 5 (14%) | 4 (4%) | .05 d | 6 (22%) | 3 (3%) | 0.002 d | 5 (30%) | 4 (3%) | .001 d |
| Delivery before gestational age 34 wks | 23 (64%) | 47 (45%) | .06 | 22 (81%) | 48 (42%) | < .0001 d | 14 (82%) | 56 (45%) | .004 d |
| Delivery before gestational age 37 wks | 36 (100%) | 102 (97%) | .57 | 27 (100%) | 111 (97%) | 1.00 | 17 (100%) | 121 (98%) | 1.00 |
| Latency from PPROM to amniocentesis, h | 4 (2–170) | 4 (1–138) | .86 | 4 (2–170) | 4 (1–138) | .14 | 4 (2–170) | 4 (1–138) | .38 |
| Latency from PPROM to delivery, h | 50 (4–624) | 47 (5–768) | .74 | 96 (5–624) | 43 (4–768) | .01 d | 63 (5–624) | 47 (4–768) | .32 |
| Maternal CRP levels at admission, mg/L | 8.0 (0–81.5) | 4.9 (1–59.4) | .10 | 11.3 (0–81.5) | 4.7 (0–47.5) | < .0001 d | 22.2 (2.4–81.5) | 4.8 (0–59.4) | < .0001 d |
| WBC count at admission, × 10 9 L, median | 13.0 (6.1–24.3) | 12.2 (7.8–26.9) | .24 | 13.3 (9.5–24.3) | 12.3 (6.2–26.9) | .03 d | 13.7 (9.5–24.3) | 12.3 (6.2–36.9) | .03 d |
| Administration of corticosteroids | 27 (75%) | 55 (52%) | .03 d | 24 (89%) | 58 (51%) | < .0001 d | 16 (94%) | 60 (48%) | .001 d |
| Administration of antibiotics | 35 (97%) | 97 (92%) | .31 | 27 (100%) | 105 (92%) | .13 | 17 (100%) | 115 (93%) | .25 |
| Induction of labor | 15 (42%) | 18 (17%) | .003 d | 10 (37%) | 23 (20%) | .07 | 6 (35%) | 27 (22%) | .22 |
| Spontaneous vaginal delivery | 29 (81%) | 70 (67%) | .12 | 22 (81%) | 77 (68%) | .15 | 13 (76%) | 86 (69%) | .55 |
| Birthweight, g | 1912 ± 733 | 2124 ± 668 | .11 | 1560 ± 695 | 2191 ± 632 | < .0001 d | 1518 ± 755 | 2146 ± 647 | < .0001 d |
| Apgar score <7 at 5 min | 3 (8%) | 1 (1%) | .02 d | 3 (11%) | 1 (1%) | .004 d | 3 (18%) | 1 (1%) | < .0001 d |
| Apgar score <7 at 10 min | 1 (3%) | 1 (1%) | .42 | 2 (7%) | 0 (0%) | .003 d | 1 (6%) | 1 (1%) | .10 |
a The P value is the comparison between groups with and without microbial invasion of the amniotic cavity
b The P value is the comparison between groups with and without intraamniotic inflammation
c The P value is the comparison between groups with and without microbial-associated intraamniotic inflammation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


