Vaginal and Transdermal Estrogen-Progestin Contraception
The more options available for contraception, the more effective family planning is within a society. Vaginal and transdermal estrogen-progestin contraception have considerable appeal for some women unsatisfied with other methods. The use of vaginal contraception in the United States has steadily increased since its introduction, and it is now one of the more popular methods. An important advantage of vaginal and transdermal steroid contraception is an improvement in compliance achieved by the elimination of a daily regimen of treatment. Although not yet documented by epidemiologic studies, it is expected that vaginal and transdermal steroid contraception will be associated with the same characteristics, benefits, and problems seen with oral contraceptives.
Vaginal Estrogen-Progestin Contraception
The vagina provides an ideal site for contraceptive steroid absorption. The stratified squamous epithelium, unlike the skin, is not cornified, permitting easier steroid penetration to the underlying lamina propria, made of collagen and elastin, which is richly vascularized. A muscular layer under the epithelium has smooth muscle fibers running in both circular and longitudinal directions. The final layer of areolar connective tissue contains a second vascular plexus. There are no fat cells, glands (secretions are transudates, not glandular), or hair follicles to interfere with drug absorption.1
Like subcutaneous, intramuscular, transdermal, and intrauterine contraceptive methods, the vaginal route avoids gastrointestinal absorption and the first-pass liver effect. Absorption from the gastrointestinal tract can be unpredictable and may be compromised by vomiting, drug-drug interference, or decreased intestinal absorption capacity. The gastrointestinal lumen and the liver are sites of elimination for many compounds, and avoidance of the first-pass effect is particularly advantageous for compounds that undergo a high degree of hepatic metabolism; orally administered natural estrogens, for example, are 95% metabolized by the liver. Oral administration results in marked fluctuations of contraceptive steroid serum concentrations that may
lead to side effects like irregular bleeding and nausea. These daily changes are lower with vaginal than with oral or transdermal administration and lowest with implant and intrauterine methods.1,2
lead to side effects like irregular bleeding and nausea. These daily changes are lower with vaginal than with oral or transdermal administration and lowest with implant and intrauterine methods.1,2
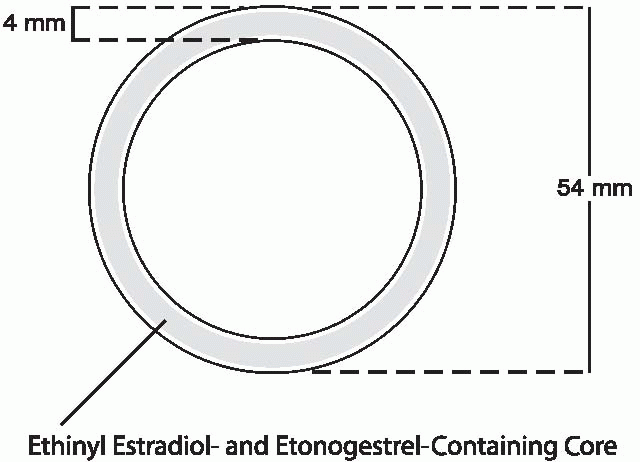
Vaginal contraceptive rings have been studied for 35 years. Six progestin-only and seven different estrogen-progestin (combined) vaginal contraceptive rings have been designed to provide 1 week to 1 year of contraception with weaker progestins like progesterone and medroxyprogesterone in short-acting rings (1 week) and more potent levonorgestrel and nestorone in long-acting ones (up to a year). Only the “NuvaRing” vaginal combined steroid contraceptive is available in the United States. It is a flexible, soft, transparent ring made of ethylene vinyl acetate copolymer in which are contained crystals of etonogestrel (the biologically active metabolite of desogestrel, previously known as 3-ketodesogestrel) and ethinyl estradiol. This ring is covered with a 2-µm-thick membrane of ethylene vinyl acetate. The ring is available in only one size, 4 mm in thickness and 54 mm in diameter (smaller than a diaphragm), that fits all women.
A vaginal ring that is not yet marketed delivers 15 µg ethinyl estradiol and 150 µg nestorone daily and is intended to be effective for a year with periodic removals to induce withdrawal bleeding.3,4 Rings that contain only progesterone or nestorone are being developed for use in breastfeeding women.
The Vaginal Ring Method
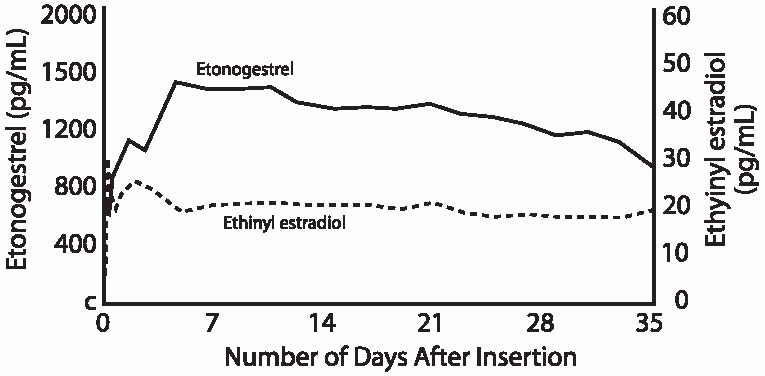
The NuvaRing releases 15 µg ethinyl estradiol and 120 µg etonogestrel per day.5 Because the progestin and the estrogen are mixed in the ethylene vinyl acetate core, in the unlikely event of damage to the ring, leakage or higher release of the hormones does not occur. Circulating estrogen and progestin levels reach target concentrations within 24 hours. The circulating estrogen levels reach a maximum level after 2 to 3 days, etonogestrel reaches maximum level after 7 days, and remains stable for 35 days.2 The ring is inserted by the patient and worn for 3 weeks. The vaginal ring can be initiated in the same fashion as oral contraceptives, on the first day of menses, a Sunday start, or an immediate, same-day start. Routine use requires the insertion of a new ring every 4 weeks to allow for withdrawal bleeding, but an acceptable and easier method is to insert a new ring on the first of every month. Continuous use is obviously an appropriate option.6 Because the ring contains enough steroid hormone to inhibit ovulation for at least a total of 5 weeks,7 when used continuously, a new ring can be inserted every 5 weeks. Breakthrough bleeding with continuous use is effectively managed by a 4-day hormone-free interval.8
 |
The ring produces circulating progestin and estrogen levels that are only 40% and 30%, respectively, of the peak levels associated with an oral contraceptive containing 150 µg desogestrel and 30 µg ethinyl estradiol.2 These levels effectively inhibit ovulation, providing pregnancy rates of less than 1% in clinical trials.5,7,9,10 If the vaginal ring is removed and not replaced within 3 hours, the manufacturer recommends backup contraception until the ring has been in place for 7 days.
 |
Clinical Responses
Taking into account bioavailability as influenced by protein binding, systemic exposure to etonogestrel is similar comparing the vaginal ring to an oral contraceptive containing 150 µg desogestrel; however, the systemic exposure to ethinyl estradiol is about 50% of that of an oral contraceptive containing 30 µg ethinyl estradiol.2 Vaginal administration is associated with the lowest estrogen exposure as measured by “area under the curve,” compared with oral and transdermal methods.11 This may explain the low incidence of estrogen-related side effects such as dysmenorrhea, nausea, and
breast tenderness.5,9,12 In a randomized comparison of the vaginal ring and the transdermal patch, more women preferred the vaginal ring because of a lower rate of side effects.13
breast tenderness.5,9,12 In a randomized comparison of the vaginal ring and the transdermal patch, more women preferred the vaginal ring because of a lower rate of side effects.13
Breakthrough bleeding and spotting rates are lower (around 6%) when compared with an oral contraceptive containing 30 µg of ethinyl estradiol and much lower than with 15 or 20 µg pills.9,14,15 Bleeding and spotting occur more frequently with extended or continuous regimens.6 The low serum steroid concentrations do not produce significant changes in LDL or HDL levels but do increase triglycerides substantially and sex hormone-binding globulin levels markedly.16 There are no clinically significant changes in clotting parameters. The vaginal ring and oral contraceptives have similar metabolic effects.17 The vaginal ring has no effect on insulin sensitivity, and no clinically significant adverse changes in carbohydrate and lipid metabolism were observed in vaginal ring users with type 1 diabetes mellitus.18,19
It is not necessary to place the vaginal ring in a specific position; it need only be in contact with vaginal mucosa and need not surround the cervix. The vaginal ring is intended to be placed in a normal vagina; infections and anatomic abnormalities are reasons for clinicians and patients to consider other methods. The most common reasons for discontinuation (about 2% to 4% in the clinical trials) have been vaginal discomfort, unwanted awareness of the ring’s presence, coital problems, or expulsion (during a year of use about 2% to 3% of women experience spontaneous expulsion). Women report that the ring is easy to insert and remove, and, although about 15% of women and 30% of partners report feeling the ring during intercourse, this is not a common reason for discontinuation.9,20 Removal for sexual intercourse is not recommended, but efficacy is maintained if the ring is replaced within 3 hours. Postmarketing surveillance studies documented a high rate of satisfaction with good cycle control.21,22
Cervical cytology and the vaginal flora are not affected by the presence of the ring.9,23,24 Despite no change in inflammatory vaginal flora, one well-done study reported that ring users have slightly more vaginal wetness (not sufficient to cause discontinuation), perhaps due to an increase in lactobacilli.25
Vaginally applied antifungal agents (miconazole) have no effect on absorption of contraceptive steroids released by the vaginal ring, nor does the use of tampons.9,26,27 Amoxicillin and doxycycline do not affect serum estrogen or progestin levels or the steroid pharmacokinetics associated with the vaginal ring.28
The spermicide, nonoxynol-9, has no effect on the release and absorption of the hormones in NuvaRing, as assessed by the measurement of serum levels of ethinyl estradiol and etonogestrel.26 Combining a barrier method that contains nonoxynol-9 should not affect the contraceptive efficacy of the ring. However, spermicides do not provide protection against sexually transmitted infections, and there is no good clinical reason to combine the ring with a spermicide.
Summary of Vaginal Ring Advantages
Elimination of a daily or coital regimen, resulting in better compliance.
Avoidance of gastrointestinal absorption problems.
Avoidance of first-pass liver effects.
Forgiving of delays; contraceptive efficacy for 5 weeks.
Lower systemic estrogen exposure; lower incidence of estrogen side effects.
Less frequent breakthrough bleeding and spotting.
Transdermal Estrogen-Progestin Contraception
The transdermal contraceptive patch (Ortho-Evra) has an area of 20 cm2 (4.5 × 4.5 cm) and three layers in a matrix-type arrangement. The backing outer polyester layer provides support for the middle layer that contains the adhesive and the hormones, and the inner layer is a polyester liner that is removed from the adhesive layer just before application. The size is that required to deliver an effective dose of the steroid hormones. The patch contains 750 µg ethinyl estradiol and 6 mg of norelgestromin and delivers 20 µg ethinyl estradiol and 150 µg norelgestromin each day when applied to discrete locations on the lower abdomen, upper outer arm, the buttock, or the upper torso (excluding the breast). Norelgestromin is the primary active metabolite of orally administered norgestimate and was previously known as 17-deacetylnorgestimate. Norelgestromin still undergoes liver metabolism with transdermal application; however, the resulting metabolite, levonorgestrel, is highly bound to sex hormone-binding globulin, limiting its biologic impact. About 97% of norelgestromin is bound to albumin and 3% is unbound.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


