Mesenchymal tumors
Mixed epithelial and mesenchymal tumors
Endometrial stromal and related tumors
Carcinosarcoma
Endometrial stromal sarcoma, low grade
Adenosarcoma
Endometrial stromal nodule
Carcinofibroma
Undifferentiated stromal sarcoma
Adenofibroma
Smooth muscle tumors
Adenomyoma
Leiomyosarcoma
Atypical polypoid variant
Epithelioid variant
Myxoid variant
Smooth muscle tumor of uncertain malignant potential
Leiomyoma, not otherwise specified
Histologic variant
Mitotically active variant
Cellular variant
Hemorrhagic cellular variant
Mesenchymal tumors: The pure mesenchymal tumors can be further classified into endometrial stromal sarcoma (ESS), leiomyosarcoma (LMS) – including the epithelioid and myxoid variants – and undifferentiated endometrial/uterine sarcoma (UUS) according to the cell of origin.
Historically, uterine carcinosarcoma was classified as a type of uterine sarcoma and was termed malignant mixed Müllerian tumor (MMMT) or mixed mesodermal sarcoma. However, these neoplasms are now classified as carcinomas since they derive from a monoclonal neoplastic cell, which has more characteristics of epithelial than stromal neoplasms. In addition, the epidemiology, risk factors, and clinical behavior associated with carcinosarcoma suggest a closer relationship to endometrial carcinoma than to sarcoma [6–8]. Despite this, and probably because it behaves more aggressively than the ordinary endometrial carcinoma, carcinosarcoma is still included in most retrospective studies of uterine sarcomas, as well as in WHO 2003 classification [1].
ESSs have traditionally been divided into low-grade and high-grade tumors. However, as high-grade ESS often lack evidence of endometrial stromal cell differentiation and are clinically more aggressive, it has been proposed that they should be classified as undifferentiated endometrial or uterine sarcoma. Thus, ESS are by definition now considered all “low-grade” sarcomas and undifferentiated sarcoma is now the preferred terminology for all “high-grade” ESS [1, 5, 6]
Epidemiology
Histopathological criteria for uterine sarcomas have greatly evolved over the last few years. As per a large series with 127 cases including carcinosarcomas, the most common uterine sarcoma were leiomyosarcoma and carcinosarcoma [9] (Fig. 30.1). However, leiomyosarcomas and endometrial stromal sarcomas accounts for 66–23 %, respectively, of all uterine sarcomas after excluding carcinosarcomas, with all of the other subtypes being exceedingly rare. According to most recent and largest series (n = 419) in Norway on uterine sarcomas from 1970 to 2000 using 2003 WHO classification by Abeler and colleagues (excluding carcinosarcomas), 62 % were leiomyosarcomas, 20 % were ESSs, and 18 % were rare subtypes including undifferentiated sarcomas (6 %), adenosarcomas (5.5 %), sarcoma, not otherwise specified (NOS) (4.5 %), rhabdomyosarcoma (<!%), giant cell sarcoma (<!%), and perivascular epithelioid cell tumor (PEComa; <1 %) [10].
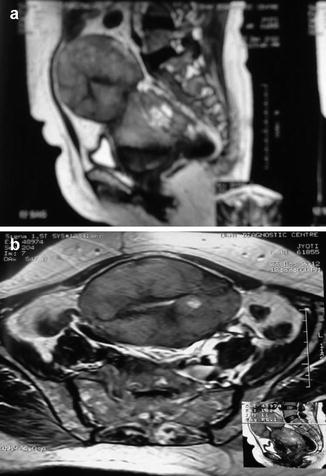

Fig. 30.1
A 60-year-old woman with carcinosarcoma. (a), Sagittal and (b), coronal T2-weighted MR image of pelvis shows heterogeneous endometrial mass with areas of necrosis
According to a published series, the median ages reported ranges from 48 to 57 years for leiomyosarcoma, 57 to 67 years for carcinosarcomas, 42 to 51 years for ESSs, and 58 to 66 years for adenosarcomas [5]. In a review of 208 patients with leiomyosarcomas from the Mayo clinic, only 41 % were postmenopausal [11]. Thus, many patients with carcinosarcoma are postmenopausal at the time of diagnosis, whereas patients with leiomyosarcoma, ESS, or undifferentiated sarcoma may be pre- or postmenopausal at the time of diagnosis. This would have potential implications in terms of fertility for these patients.
Genetic factors have been suggested to play a role, as incidence is twice as high among black women compared to white women. This race-specific incidence pattern is reversed in endometrial carcinoma, in which the incidence among white women is three times higher than that among black women [5, 12]. In a series by Zelmanowicz et al., women with carcinosarcoma are more likely to be of African American descent than those with endometrial adenosarcomas (among 453 patients and controls, 28 % versus 4 %, p = 0.001) [8]. Then, Brooks et al. reported that the age adjusted incidence of uterine sarcomas in African American women was twice that of controls. The age-adjusted incidences of leiomyosarcomas for black and white women, respectively, were 1.5 and 0.9 per 100,000. Similarly, those for carcinosarcoma in black and white women, respectively, were 4.3 and 1.7 per 100,000 [13].
Etiology and Risk Factors
The development of the basic understanding of uterine sarcoma has been slow. The majority of cases are felt to be sporadic, with no specific etiology, and most have complex karyotypes [14]. However, specific chromosomal translocations have been identified in an increasing number of uterine sarcomas, resulting in fusion genes that are constitutive and involve activation of transcription factors [5, 15].
Genetics of Uterine Sarcomas
ESS has specific somatic mutations that have been discovered by cytogenetic studies namely, fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR) analyses. In the database of chromosome aberrations in cancer by Mitelman, the cytogenetic features t (7;17) (P15;q11), t (6;7) (P21, P15), and t (6,10) (P21;P11), and molecular genetic features (JAZF1/ SUZ 12), (JAZ F1/PH F1), and (EPC1/PH F1 fusion gene) of ESS, are recorded [16].
Undifferentiated endometrial sarcomas lack these JAFZ1 based rearrangements but instead appear to frequently harbor the YWHAE-FAM22A/B genetic fusion and is considered to be specific for these tumors [16].
LMP2-deficient mice are reportedly prone to spontaneous development of uterine LMS and histopathological experiments demonstrated a high correlation between a loss of LMP2 and malignancy of uterine tumors developing in myometrium. Recent reports have also shown a loss in the IFN-γ-inducible expression of LMP2 in a LMS culture cell line. Also, histopathological examinations with IFN-γ-deficient mice revealed that the IFN-γ pathway is especially required for LMP2 expression in normal myometrium. Therefore, organ-specific LMP2 functions might be one of the factors involved in sarcogenesis of uterine leiomyosarcomas [17].
Cowden and colleagues analyzed carcinosarcomas for the presence of somatic mutations. The rate of mutations identified for each gene analyzed was PIK3CA (56 %), KRAS (44 %), TP53 (33 %), and CTNNB1 (6 %). Tp53 mutation was the only mutational event that retained an independent association with survival. Also, the Akt/b-catenin pathway and alteration in Rb expression have been suggested to be involved in the development of carcinosarcomas [18].
Prior Radiation Therapy
Although prior exposure to pelvic radiotherapy is considered a risk factor primarily for carcinosarcoma and undifferentiated sarcoma, this is rarely an etiological factor for leiomyosarcoma or STUMP.
In a large series from Mayo clinic of 208 leiomyosarcoma cases, only 1 woman (0.6 %) was exposed to prior pelvic radiation [11]. MD Anderson Cancer Center described 41 cases of STUMP and found that none of them had a history of prior radiation exposure [19]. Christopherson et al. described 2 cases among 33 patients with leiomyosarcoma who had a history of pelvic radiation [20].
On the other hand, Norris at al described in his series that 29 % of carcinosarcoma (9 of 31 patients) had received pelvic radiotherapy 7–26 years prior to diagnosis [21]. Among 1,208 patients of uterine malignancies reported by Meredith et al. in 1986, 30 patients (2.4 %) had received prior radiation and 5 (17 %) among them developed carcinosarcoma, for a crude association of 11 %. It has been concluded that postradiation carcinosarcoma occur at a younger age than those arising de novo; however, latency to diagnosis of malignancy is shorter in older age group [22].
Pothuri et al. compared clinicopathological characteristics of 23 cases of uterine malignancies occurring after prior radiation therapy to 527 cases of uterine cancers arising de novo [23]. Carcinosarcomas and undifferentiated sarcomas accounted for 9 (39 %) of the 23 radiation associated malignancies compared to only 33 (8 %) of sporadic cases. They also suggested that radiation induced malignancy tend to have worse outcome, possibly due to lack of early symptomatology.
Exposure to Hormones and Tamoxifen
An association between exposure to hormonal agents, including tamoxifen, and increased risk of uterine sarcomas has been suggested.
As per the data from Finish Cancer registry, uterine sarcomas occurred in 76 out of the 243,857 women who were identified as having used estradiol-progestin therapy for more than 6 months [24]. The ever use of estradiol-progestin therapy was associated with 60 % elevation in the risk for any uterine sarcoma, mostly for leiomyosarcoma (standardized incidence ratio [SIR], 1.8; 95 % CI, 1.3–2.4), but not for endometrial stromal sarcoma (SIR, 1.4; 95 % CI, 0.9–2.1). Also, this elevated risk was only noted in women who had used estradiol-progestin therapy for 5 years or more. However, despite a possible increased risk, the overall absolute risk of uterine sarcomas is still exceedingly low.
Recently, similar to endometrial adenocarcinoma, a possible association between tamoxifen use among breast cancer patients and increased risk for uterine sarcomas, particularly carcinosarcomas, has been reported and is significant with more than 4 years of tamoxifen use. In a study by Hoogendoorn et al. [25], among patients diagnosed with uterine cancer, carcinosarcomas accounted for a larger proportion of cases in those who had received prior tamoxifen therapy compared to those who had not (15 % vs. 4 %, respectively).
Hereditary Predisposition
Hereditary predisposition to certain uterine sarcomas has also been suggested but still remains to be clearly elucidated. As per Danish Hereditary Nonpolyposis Colorectal Cancer (HNPCC)-register, sarcomas of various sites represented 1 % (n = 14) of 164 HNPCC families with disease predisposing mutations [26]. Three of these 14 sarcomas were uterine: one was a carcinosarcoma in a 44-year-old with loss of MSH2 and MSH6 expression in the sarcoma, other was also a carcinosarcoma in a 55-year-old with loss of MSH6 expression and the third was leiomyosarcoma in a 44-year-old with loss of MSH2 and MSH6 expression. The overall risk of uterine sarcoma is still low in these hereditary syndromes.
Clinical Presentation and Diagnosis
The most common presenting symptom of uterine sarcomas is abnormal uterine bleeding (pre- or postmenopausal bleeding), and is nearly universal in those with carcinosarcoma, but may occur in as few as 40 % of those with leiomyosarcomas [1, 5, 6]. (Table 30.2).
Symptom | ESS (%) | CS (%) | LMS (%) | AS (%) |
|---|---|---|---|---|
Asymptomatic | 10–15 | 5–10 | 10–14 | 10–15 |
Abnormal vaginal bleeding | 65–70 | 65–70 | 50–55 | 60–62 |
Abdominopelvic mass | 10–15 | 1–15 | 45–50 | – |
Abdominopelvic pain | 15–20 | 8–10 | 20–25 | 18–22 |
Uterine enlargement | 60–65 | – | – | – |
Uterine cavity lesion | 18–20 | – | – | – |
Vaginal discharge | 1–5 | – | – | – |
Abdominal distension | – | – | – | 1–2 |
Carcinosarcomas usually occur in an older age group; most patients being postmenopausal. The frankly malignant variants grow rapidly and at physical examination, 50–95 % of patients have enlargement of the uterus with 50 % of patients having protrusion of a polypoidal lesion through the endocervical canal. In advanced cases, presentation maybe similar to that of ovarian cancer with pleural effusion, ascites, and adnexal masses. Uterine curettage usually detects malignant tissue in the uterus, as it is a lesion that arises in the endometrium (unlike true uterine sarcoma that often does not have an endometrial component).
Uterine enlargement and a presumptive diagnosis of uterine leiomyomas, leiomyomas are nearly universal findings in patients with leiomyosarcoma. They usually present in women above 40 years of age and majority of these tumors arise de novo, with less than 5 % arising from malignant transformation of an existing leiomyoma. Occasionally, the presentation may be that of hemoperitoneum due to tumor rupture, extrauterine extension, or metastases such as persistent cough, back pain, and ascites [27] Berchuck et al. reported that in 14 patients with leiomyosarcomas undergoing dilatation and curettage, a prehysterectomy diagnosis was made in only 8 (31 %).
Endometrial stromal sarcoma usually presents between 40 and 55 years of age. Again, the most common presenting symptom is abnormal uterine bleeding with some women presenting with pelvic pain and/or dysmenorrhea but as many as 25 % of them may be asymptomatic. In a report by Memorial Sloan-Kettering Cancer Center, endometrial stromal sarcoma was diagnosed as an incidental finding in 42 % of cases [28]. Some cases have been reported in women with ovarian polycystic disease, after estrogen use, or tamoxifen therapy. At presentation, extrauterine pelvic extension, most commonly involving the ovary, is found in up to one-third of patients. Thus, when evaluating an ovarian tumor microscopically consistent with an endometrial stromal tumor, it is important to exclude a prior history of uterine endometrial stromal tumor and to suggest inspection of the uterus, as the latter are far more common.
Preoperative endometrial assessment with either office Pipelle or dilation and curettage (D&C) under anesthesia are limited in the evaluation and correct diagnosis of uterine sarcomas; however, it is the investigation of choice in women who presents with abnormal uterine bleeding. Diagnosis of carcinosarcoma is usually confirmed, or at least suggested, at the time of endometrial assessment, but leiomyosarcomas are rarely diagnosed before hysterectomy. In a series by Bansal et al. [29], invasive tumor was diagnosed in 86 % cases of uterine sarcomas on preoperative endometrial sampling and 64 % out of them had correct histology as compared with final histopathology. However, 70 % of cases in his series were carcinosarcomas with only four leiomyosarcomas, two endometrial sarcomas, and eight other sarcomas diagnosed on final histology. Diagnosis of carcinosarcoma may be missed because endometrial biopsy/curettage does not adequately sample both the epithelial and stromal components of the tumor; however, appropriate preoperative referral is usually made because of the presence of a malignancy, and staging is accomplished at the time of hysterectomy. Conversely, most leiomyosarcomas are diagnosed after hysterectomy at the time of histological review of the surgical specimen. This has been highlighted in a GOG study reported by Major et al. [30] where fewer patients with leiomyosarcoma were referred to a higher centre than those with carcinosarcoma (301 carcinosarcomas, 59 leiomyosarcomas, and 93 endometrial stromal sarcomas).
Imaging Perspective
Based on imaging findings, the preoperative diagnosis of uterine sarcomas and the distinction among the various histologic subtypes is challenging.
MRI is usually a preferred modality for assessing carcinosarcoma because of its improved ability to characterize the depth of the lesion, delineate local extrauterine spread of disease as well as assessing for the presence of necrosis and hemorrhage (please see chapter on “Imaging”).The tumor generally appears as areas of heterogeneous high intensity on T2-weighted magnetic resonance (MR) images and low intensity on T1-weighted images (Fig. 30.1). CT is preferred for staging and assessment of distant metastases and nodal involvement.
The appearance of leiomyosarcoma is variable on MRI and no preoperative imaging can reliably differentiate between leiomyoma and leiomyosarcoma. They usually appear as large heterogeneously enhancing uterine masses, with central T2 hyperintensity indicative of necrosis. Role of CT scan has been limited to identifying extrauterine disease, including local spread and metastasis. There is low incidence of adnexal or pelvic lymph node involvement. The most common site of metastasis is the lungs followed by liver and other abdominal sites, thus CT chest is usually indicated for evaluation.
ESS appears as a polypoidal lesion with low T1 signal and heterogeneously increased T2 signal . Because of its tendency for lymphatic and vascular invasion, ESS may show a “bag of worms” appearance, especially in cases of high grade tumors.
Tumor Markers
There is no reliable tumor marker for diagnosis of uterine sarcomas. Serum CA-125 is seen to be increased in 17–33 % of leiomyosarcomas, ESSs, and carcinosarcomas [9]. However, it should not be used routinely in the evaluation and diagnosis of these tumors. Recently, serum lactate dehydrogenase has been found to be an interesting addition to imaging in the evaluation of uterine leiomyosarcomas. In a series by Goto et al. [31], combining serum LDH and dynamic MRI has been found to have accuracy of 99.3 % in predicting uterine leiomyosarcomas. These results are impressive and may be of help in evaluating women with presumed benign leiomyoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


