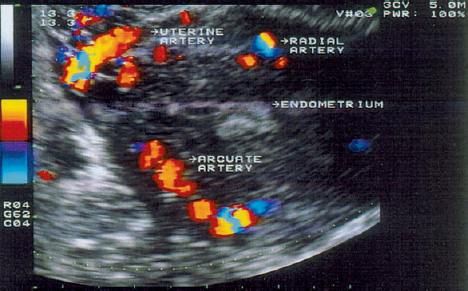
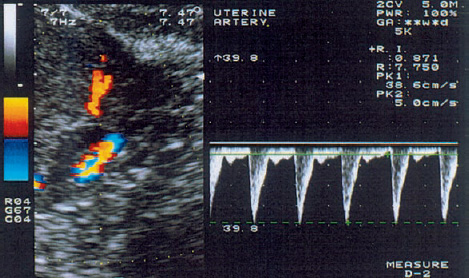
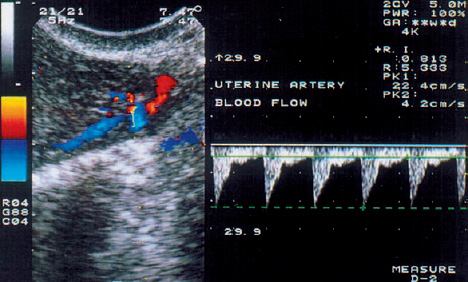
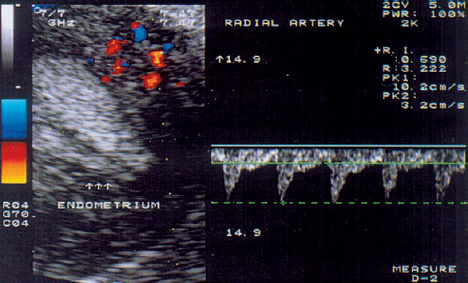
3 Uterine Blood Flow in Fertile and Infertile Women Anatomy. The uterus derives most of its blood supply from the uterine arteries, with a small amount supplied by the ovarian arteries. The uterine arteries branch into the arcuate arteries (Fig. 3.1), which project circumferentially into the outer third of the myometrium. The arcuate arteries divide further into the radial arteries, which then branch into the basal arteries and spiral arteries after crossing the myometrial–endometrial boundary. The relatively short basal arteries terminate in a capillary bed that supplies the stratum basale of the endometrium. The spiral arteries project farther into the endometrium and open into a broad capillary bed that supplies the stratum functionale of the endometrium. Menstrual cycle. Interestingly, only the spiral arteries show demonstrable anatomical changes during the menstrual cycle24. These arteries contract when menstruation occurs, possibly as a result of falling estrogen and progesterone levels. This vasoconstriction induces local hypoxia, ischemia, and cell death in the stratum functionale. The distal portion of the arteriolar vascular bed and the capillary bed are then sloughed along with the stratum functionale. The basal arteries do not react to falling estrogen and progesterone levels and thus serve to maintain the integrity of the stratum basale during the menstrual cycle12. A 3-fold to 5-fold increase in endometrial thickness occurs during the next menstrual cycle. To ensure this, a completely new capillary bed arises from the rapidly growing proximal portions of the spiral arteries. The process begins with the growth of new capillaries from the stratum basale12. A new intima and muscular coat develops in the capillary walls20. Implantation. The angiogenesis that occurs during the proliferative and secretory phases of the menstrual cycle serves to prepare the endometrium for the implantation of a fertilized ovum13. After fertilization has occurred, implantation begins with the attachment of the blastocyst to the endometrial surface. As the trophoblastic cells invade the endometrium, maternal capillaries are breached, causing profound physiological and structural changes to occur in the uterine vascular bed21. One of the first changes is increased vascular permeability at the implantation site3. This is followed by a metabolic activation of the endometrium in preparation for angiogenesis3. Subsequent placentation is facilitated by the changes in the maternal vascular bed. Further details on this process can be found in the section on the Doppler examination of early placentation and embryonic blood flow (p. 103). Fig. 3.1 Transvaginal image of an arcuate arterial network. More than any other available examination technique, transvaginal color Doppler sonography requires an accurate knowledge of the location of the uterus, myometrium, endometrium, and the vessels that supply them5,11,17,18. It is known that uterine perfusion depends largely: on the age of the patient, the phase of the menstrual cycle, and other special factors such as pregnancy or neoplasia19. These factors will be analyzed below in detail. Complex relationships exist between ovarian hormone levels in the peripheral venous blood and the blood flow parameters in the uterine arteries2,8,9,23. During the proliferative phase, a small amount of end-diastolic blood flow can be detected in the uterine arteries in the majority of women (Fig. 3.2). The resistance index (RI) until day 13 of a 28-day cycle is in the range 0.88 ± 0.04. Steer et al.23 reported that diastolic flow was no longer detectable in the uterine arteries on the day of ovulation. Goswamy and Steptoe8 found an increasing resistance index and systole/diastole ratio during the postovulatory fall in serum estradiol levels. Increased resistance in the uterine arteries was measured three days after the surge in luteinizing hormone (LH), and Scholtes et al.22 found that the pulsatility index (PI) in the uterine arteries was highest on day 16 of the menstrual cycle. Fig. 3.2 The uterine artery appears to the left of the cervix at the junction of the cervix and uterine corpus. The blood flow velocity in the uterine artery during the proliferative phase is characterized by low end-diastolic blood flow and a high vascular resistance (RI = 0.87). These results could be due to increased uterine contractility10 and increased compression of the vessels traversing the uterine wall, thus reducing the vessel diameters and causing greater flow resistance. During the normal menstrual cycle, a sharp rise of end-diastolic blood flow velocities can be demonstrated between the proliferative and secretory phases18. It is particularly noteworthy that the lowest vascular resistance coincides with the period of maximal corpus luteum function (RI = 0.84 ± 0.04), when implantation most commonly occurs (Fig. 3.3). It seems logical that the blood flow to the uterus is highest in the luteal phase, and this increase has been reported by Kurjak et al.18, Goswamy et al.8,9, Steer et al.23, and Bataglia et al.1. The persistence of a low RI in the luteal phase suggests that the relaxation effect on the uterine arteries continues until the start of menstruation. Zaidi et al.27 described a circadian rhythm in uterine artery blood flow, independent of fluctuating hormone levels. Blood flow fluctuations similar to those in the main trunk of the uterine artery were observed in the radial and spiral arteries following the introduction of transvaginal color Doppler and pulsed Doppler sonography14 (Fig. 3.4). Our results show that the postovulatory fall of serum estradiol levels coincides with a postovulatory rise of the RI in the myometrial vessels (Fig. 3.5). Fig. 3.3 The blood flow velocity in the uterine artery during the secretory phase is characterized by a higher velocity and lower resistance index (RI = 0.81). Fig. 3.4
Uterine Blood Supply
Changes in Uterine Blood Flow during the Menstrual Cycle
Blood Flow Parameters in the Uterine Arteries
Blood Flow Parameters in the Radial and Spiral Arteries
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree