Fig. 37.1
Insertion of the membrane on the placenta – “T” sign indicated monochorionicity and “λ” sign indicated dichorionicity
Dichorionic diamniotic (DCDA) twins form when splitting takes place by the third day after fertilization, i.e., at the two-cell stage. This occurs in almost all cases of dizygotic twins and in 25 % of monozygotic twins. If the split occurs at the early blastocyst stage between days 4–8, then monochorionic–diamniotic twins (MCDA) are formed, and if splitting occurs between days 8–12, which is the late blastocyst stage, then monochorionic–monoamniotic (MCMA) twins are formed. If the split occurs after day 13, conjoined twins result [1].
Incidence and Definition
Fifteen percent of spontaneous twin pregnancies and almost 5 % of the medically assisted twin pregnancies are monochorionic–diamniotic (MCDA) [1].
Fifteen to twenty percent of monochorionic–diamniotic twin pregnancies are complicated by twin-to-twin transfusion syndrome (TTTS) [1].
Twin-to-twin transfusion syndrome (TTTS) is a specific complication of monochorionic pregnancy resulting from an imbalance in the blood flow between the twins sharing the placenta, leading to unequal distribution of the blood flow such that one twin is compromised and the other is favored. Blood from one twin (the donor) is “transfused” into the other twin (the recipient) via connecting blood vessels within their common placenta. It has life-threatening effects upon both twins with perinatal mortality approaching 90 % if untreated [2–4].
Pathophysiology
Differential cord insertion of the MCDA twins with unequal sharing of the placental disk forms the basis of TTTS. Inter-twin vascular anastomoses as shown in Fig. 37.2 are present in virtually all monochorionic pregnancies. Arteriovenous anastomoses (labeled 1 in Fig. 37.2) with unidirectional flow, arterioarterial (labeled 2 in Fig. 37.2), or venovenous anastomoses with bidirectional flow are seen in the placenta of MCDA twins. Arteriovenous anastomoses are usually situated deeply in the placenta. Bidirectional anastomoses (arterioarterial, venovenous – both usually located on the placental surface) can assist in regulating unbalanced, unidirectional flow which occurs through the arteriovenous anastomoses and are protective against TTTS. Thrombosis of the superficial vessels may give rise to TTTS [2–4] (Fig. 37.3).
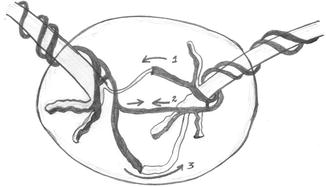
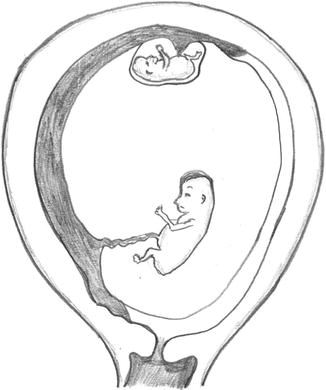

Fig. 37.2
Dark shaded vessels – veins; light shaded vessels – arteries

Fig. 37.3
Twin-to-twin transfusion syndrome (TTTS)
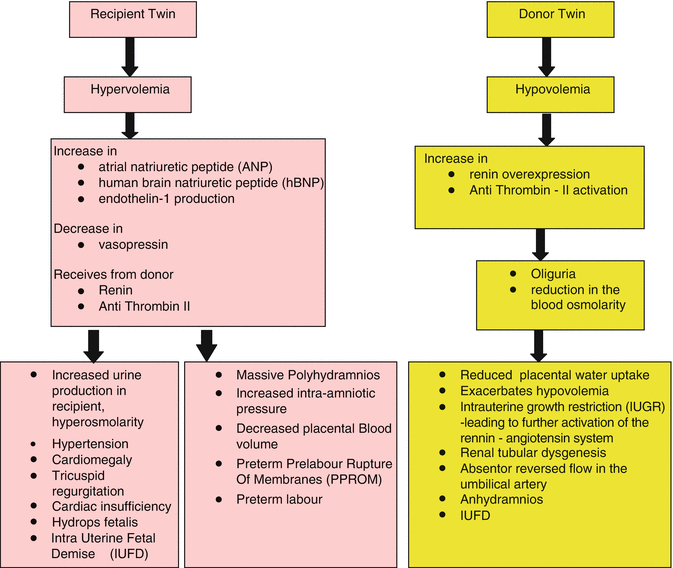
The asymmetric, bidirectional inter-twin exchange of blood and biochemical components that occurs between the donor and recipient twins results in severe hemodynamic, osmotic, and physiologic changes in both twins resulting in renal structural and functional aberrations in the donor twin and cardiovascular compromise in the recipient twin as depicted in the flowchart below [5].

Prenatal Diagnosis
Characteristic ultrasound findings enable prenatal diagnosis of TTTS. With advances in first trimester ultrasound and the widespread use of first-trimester screening, affected pregnancies are becoming increasingly diagnosed at earlier gestational ages. The first step in prenatal diagnosis is early and accurate determination of chorionicity as shown in ultrasound (USG) Image 37.1.
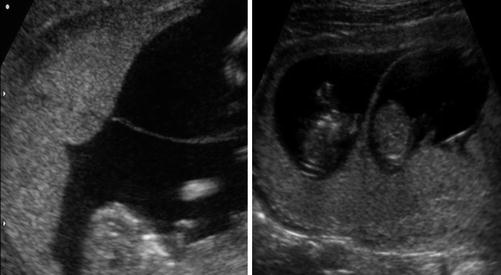

Image 37.1
Insertion of the membrane on the placenta – “T” sign indicated monochorionicity and “λ” sign indicated dichorionicity
The diagnostic criteria on ultrasound are:
In the FIRST TRIMESTER
Nuchal translucency – increased measurement – increased risk of TTTS [6]
Discordant nuchal translucency measurements (USG Image 37.2) [6]
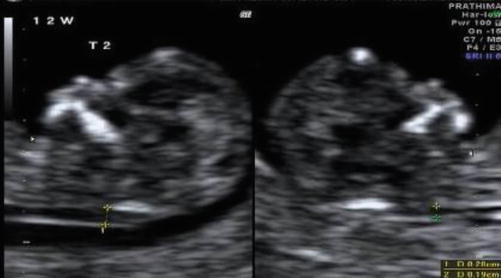
Image 37.2
Discrepant NT measurements in MCDA twins at 12 weeks
Discordant measurements of crown–rump length
Reversed “a” wave in ductus venosus in either of the twins
Placental cord insertion – differential – marginal and velamentous (USG Image 37.3)
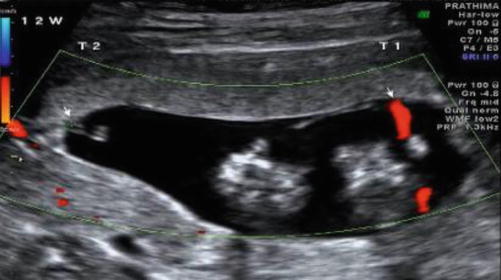
Image 37.3
Discrepant cord insertions (arrows) in MCDA twins at 12 weeks
Discordant abdominal circumference/amniotic fluid volume/bladder size
Tricuspid regurgitation in either of the twins
In the SECOND TRIMESTER
Membrane folding – fluid discordance
Discrepancy in amniotic fluid volumes – polyhydramnios–oligohydramnios sequence; significant difference in the amniotic fluid volume – deepest vertical pool (DVP) >8 cm in recipient (USG Image 37.4) and <2 cm in the donor
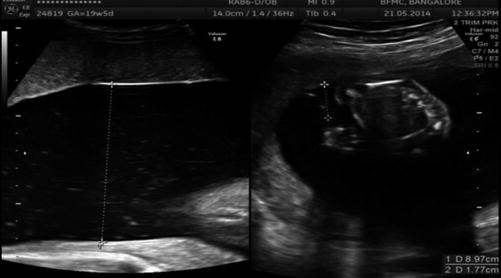
Image 37.4
Discrepant amniotic fluid in MCDA twins in 2nd trimester
Discrepancy in abdominal circumference (AC) measurement (USG Image 37.5)
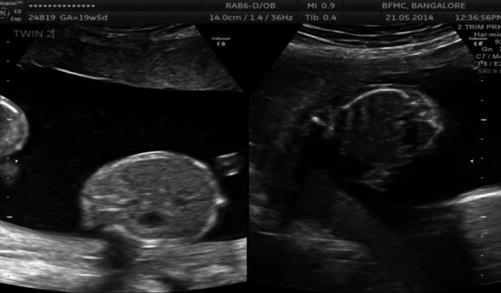
Image 37.5
Discrepant abdominal circumference in MCDA twins in the second trimester
Discrepancy in bladder size (USG Image 37.6)
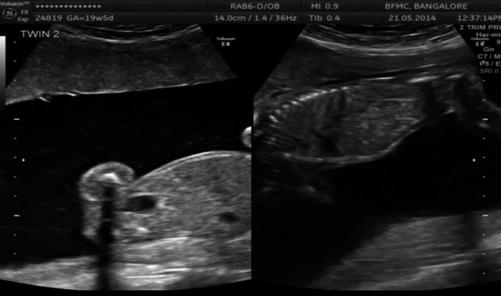
Image 37.6
Discrepant bladders in MCDA twins in the second trimester
Doppler – umbilical artery/middle cerebral artery peak systolic velocity/ductus venosus
Though all monochorionic pregnancies are considered “high risk” and need intensive surveillance, Lewi et al. have recommended a method of risk stratification of monochorionic pregnancies based on ultrasound findings.
In the first trimester, if there is discordant amniotic fluid or a discordance in crown–rump length (CRL) of ≥12 mm, these pregnancies are categorized as “high risk.” Monochorionic pregnancies with concordant amniotic fluid and difference in CRL of <12 mm are labeled as “low risk” [7].
In the second trimester (after 16 weeks), a high risk of adverse outcome was predicted by the presence of discordant amniotic fluid and discordant cord insertions. Alternatively, for cases with only discordant fluid but concordant cord insertions, adverse outcome was predicted by an inter-twin difference in abdominal circumference of >6 mm and for cases with concordant fluid but discordant cords by a difference in abdominal circumference >13 mm. Finally, in the absence of both discordant fluid and cord insertions, an adverse outcome was predicted by a difference in abdominal circumference >24 mm [7].
The “high-risk” pregnancies have >70 % risk of adverse outcome and <70 % survival rate unless treated. They will need weekly surveillance in a tertiary care center and have to be offered invasive fetal therapy [7].
The “low-risk” pregnancies have a 90 % survival rate with <10 % incidence of complications. They are followed up fortnightly in a tertiary care center [7].
TTTS is a dynamic condition which may remain unchanged, progress slowly, or develop quickly with rapid deterioration in fetal well-being hence the importance of serial surveillance of MCDA twin pregnancies, preferably in a maternal fetal medicine unit.
Staging of TTTS
The Quintero staging for TTTS was proposed in 1999 and is internationally recognized. It helps in early diagnosis and appropriate referral for treatment. The main limitations are in the prediction of progression and outcome. It indirectly considers cardiovascular changes in recipient twin via the ductus venosus Doppler. The staging is as follows:
Stage I – DVP <2 cm donor, DVP >8 cm recipient
Stage II – bladder not visible in donor
Stage III – abnormal umbilical artery (UA), ductus venosus (DV), or umbilical vein (UV) Doppler
IIIa – absent EDF or reversal of EDF in UA of donor
IIIb – reversal of flow in DV of donor
IIIc – pulsatile flow in UV of recipient
Stage IV – hydrops
Stage V – demise of one or both twins [8]
The newer staging systems [Cincinnati Staging system, Cardiovascular Profile Scoring system (CVPS), Children’s Hospital of Philadelphia system (CHOP)] incorporate echocardiographic criteria in order to overcome the limitations of the Quintero staging system, but are more complex and not widely followed in clinical practice currently [9]. Quintero staging system considers the pregnancy as a whole and gives a pregnancy specific risk, whereas the newer classifications offer an individual risk to each fetus. It also minimizes inter- and intraobserver variation. It is important to remember that patients could change from one stage to another rapidly and will not necessarily follow the sequence as specified by Quintero.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


