Tuberculosis (Mycobacteria tuberculosis)
Jeffrey R. Starke
Despite important advances in its treatment over the past two decades, tuberculosis remains a major infectious disease. Approximately one third of the world’s population harbors Mycobacterium tuberculosis and is at risk for developing disease in the near or distant future. The incidence and prevalence of tuberculosis increased over the past 15 years partly due to the human immunodeficiency virus (HIV) epidemic and the prevalence of drug-resistant tuberculosis. The failure to control tuberculosis in both developed and developing countries represents one of our greatest public health failures.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Two elements determine a child’s risk for developing tuberculosis disease.1 The first is the likelihood of exposure to an individual with infectious tuberculosis, which is primarily determined by the individual’s environment. The second is the ability of the person’s immune system to control the initial infection and keep it clinically dormant. Without treatment, disease develops in 5% to 10% of immunologically normal adults with tuberculosis infection. In young children, the risk is greater; as many as 40% of those younger than 1 year with untreated tuberculosis infection develop radiographic or clinical evidence of tuberculosis disease.
About 60% of cases of childhood tuberculosis occur in infants and children younger than 5 years.2 The ages of 5 to 14 years are often called the “favored age” because children in this range may become infected, but usually have the lowest rate of tuberculosis disease. The gender ratio for tuberculosis in children is about 1:1 in contrast to adults, in whom males predominate.
Children acquire M tuberculosis from adults in their environment. Factors that increase the risk of a child being infected with M tuberculosis include (1) birth or travel/residence in a country in which tuberculosis is endemic; (2) early childhood environments with exposures to multiple high-risk caregivers, for example, some orphanages; or (3) contact with high-risk adults who have had previous residence in a jail, prison, or high-risk nursing home, and homelessness in some communities. Factors that increase the risk of developing disease once infected include age younger than 2 years, coinfection with HIV, other immunocompromising diseases or treatments, and malnutrition.3
Most children in the United States are infected with M tuberculosis in the home, but outbreaks of childhood tuberculosis centered in elementary and high schools, nursery schools, family daycare homes, churches, school buses, and stores have occurred.4 In the United States, approximately 85% of tuberculosis cases in children occur among African American, Hispanic, Asian, and Native American children.
The more recent epidemic of HIV infection has two major effects on the epidemiology of childhood tuberculosis. First, HIV-infected adults with tuberculosis may transmit the infection to children, some of whom will develop tuberculosis disease. Second, children with HIV infection are at increased risk of progressing to tuberculosis disease once infected.5 Children with tuberculosis should have HIV serotesting because the two infections are linked epidemiologically.6
Transmission of M tuberculosis is virtually always by person-to-person spread via the respiratory route. Mucous droplets become airborne when the index case coughs, sneezes, laughs, or sings. Infected droplets dry and become droplet nuclei, which remain suspended in the air for hours.
Of the several patient-related factors associated with transmission of M tuberculosis, a positive acid-fast smear of the sputum correlates most closely with infectivity.7 However, adults with a negative acid-fast sputum smear may still be contagious. Extensive epidemiologic studies show that most children with typical tuberculosis disease rarely, if ever, infect other children or adults. In the absence of cavitary lesions, which are extremely rare in childhood, the bacilli are relatively sparse in the endobronchial secretions of children with pulmonary tuberculosis. When children with tuberculosis cough, they rarely produce sputum and lack the tussive force necessary to suspend infectious particles in the air. However, adolescents with reactivation forms of pulmonary tuberculosis, particularly if they have pulmonary cavities or extensive infiltrates, may be infectious to others.
 MYCOBACTERIOLOGY AND PATHOPHYSIOLOGY
MYCOBACTERIOLOGY AND PATHOPHYSIOLOGY
Mycobacteria are nonmotile, nonspore-forming, pleomorphic, weakly gram-positive rods that are typically slender and slightly bent. The cell walls contain lipid and wax that make these organisms more resistant than most others to light, alkali, acid, and the bactericidal action of antibodies. Growth is slow with a generation time of 14 to 24 hours. Acid fastness is the hallmark of mycobacteria. Cells appear red when stained with fuchsin (Ziehl-Neelsen or Kinyoun stain), appear purple with crystal violet, or exhibit yellow-green fluorescence under ultraviolet light (auramine and rhodamine, as in Truant stain).
In more than 95% of cases, the portal of entry for M tuberculosis is the lung. Small particles are inhaled beyond the normal clearance mechanisms of the lungs and multiply initially within the alveoli and alveolar ducts. The initial inflammation with polymorphonuclear leukocytes is replaced by epithelioid cell proliferation and the appearance of giant cells with lymphocytic infiltration. Macrophages ingest the bacilli but are not able to kill them. Replication of the organisms occurs within the macrophages, which carry some of the organisms through lymphatics to the regional lymph nodes.
As the initial cycle of macrophage ingestion and replication of bacilli continues, development of cutaneous hypersensitivity and cell-mediated immunity occurs most often between 4 and 8 weeks after onset of infection. During this time, the initial focus grows larger and has not yet become encapsulated. Occasionally, this focus is visible on the chest radiograph, but the radiograph usually remains normal and the child is asymptomatic. If adequate immunity is established, the parenchymal portion of the primary complex heals completely by fibrosis and/or calcification after undergoing caseous necrosis and encapsulation.
During the creation of the parenchymal lesion and the accelerated caseation brought on by the development of hypersensitivity, the bacilli from the primary complex spread via the bloodstream and lymphatics to the apices of the lungs, liver, spleen, meninges, peritoneum, lymph nodes, bones, and joints.
In most cases of tuberculosis infection in children, the infection is held in check locally and distantly. However, in some individuals, hilar or paratracheal lymph nodes become enlarged by the host inflammatory reaction to the tubercle bacilli.8 The nodes may encroach on the regional bronchus or bronchiole. Partial obstruction caused by external compression leads to hyperinflation in the distal lung segment. Inflamed, caseous nodes may attach to the bronchial wall and erode through it, leading to endobronchial tuberculosis. Air is reabsorbed beyond this obstruction, and collapse of the segment of the lung occurs. The resulting lesion is a combination of pneumonia and atelectasis, commonly referred to as a collapse-consolidation or segmental lesion.
A fairly predictable timetable is apparent for events that may complicate the initial tuberculosis infection and complications. Massive lymphohematogenous dissemination leading to tuberculous meningitis, and miliary or disseminated disease occurs no later than 2 to 6 months after infection. Clinically significant lymph node or endobronchial tuberculosis usually appears within 3 to 9 months. Lesions of the bones and joints usually take at least a year to develop, whereas disease of the genitourinary tract may be evident 5 to 25 years after infection.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The terminology used to describe various phases of tuberculosis follows the pathophysiology of the disease. A child is in the exposure stage when the child “shares the air” with an adult with contagious tuberculosis. In this stage, there are no clinical manifestations and the tuberculin skin test remains negative. Some children in this stage ultimately develop a positive tuberculin skin test if infection takes hold. Whereas adults in this stage usually do not get treated, young children are treated because progression to disease may occur rapidly.
Latent infection with tuberculosis means that replication of M tuberculosis has occurred within the lungs and, perhaps, in other tissues. The tuberculin skin test is positive, but the chest radiograph is normal or shows only evidence of the initial infection. In addition, there are no signs or symptoms of disease. Tuberculosis disease occurs when clinical manifestations of pulmonary or extrapulmonary tuberculosis become apparent by clinical signs and symptoms, chest radiograph, or other diagnostic techniques.
Latent (Asymptomatic) Infection
The vast majority of children with tuberculosis infection develop no signs or symptoms at any time. Occasionally, the initiation of infection is marked by several days of low-grade fever and mild cough. Rarely, the child experiences a clinically significant disease with high fever, cough, malaise, and flulike symptoms that resolve within a week. These children have a reactive tuberculin skin test, and the purpose of treating them is to prevent them from developing reactivation tuberculosis in the future.
Pulmonary
The symptoms and physical signs of pulmonary tuberculosis in children are surprisingly meager, considering the degree of radiographic changes often seen.9 The physical manifestations of disease tend to differ by the age of onset. Young infants and adolescents are more likely to have significant signs or symptoms, whereas school-age children usually have clinically silent radiographic disease. More than 50% of infants and children with pulmonary tuberculosis have no physical findings and are discovered only via contact tracing of an adult with tuberculosis. Infants are more likely to experience signs and symptoms because of their small airway diameters relative to the parenchymal and lymph node changes that occur. Nonproductive cough and mild dyspnea or wheezing, especially at night, are the most common symptoms. Systemic complaints such as fever, night sweats, anorexia, and decreased activity occur less often. Some infants have difficulty gaining weight or develop a true failure-to-thrive presentation that does not improve significantly until after several months of treatment.
Pulmonary signs are even less common. Some young children with bronchial obstruction have signs of air trapping, such as localized wheezing or decreased breath sounds, that may be accompanied by tachypnea or frank respiratory distress.
In chest radiography, the hallmark of pulmonary tuberculosis in infants and children is the relatively large size of the hilar or paratracheal lymphadenitis as compared with the less significant size of the initial parenchymal focus (Fig. 269-1A).10 Hilar lymphadenopathy is almost invariably present with childhood tuberculosis, but it may not be distinct on a plain radiograph when calcification is not present. Significant atelectasis and/or pulmonary infiltrate make it impossible to discern the lymph node enlargement. Children with tuberculosis may have the radiographic picture of lobar pneumonia without impressive or specific adenopathy.
Adolescents with pulmonary tuberculosis may develop segmental lesions with adenopathy or apical infiltrates with or without cavitation that are typical of adult reactivation tuberculosis (Fig. 269-1B). Regional lymphadenitis is absent in the latter type of disease.
The course of thoracic lymphadenopathy and bronchial obstruction can follow several paths if antituberculosis chemotherapy is not given. In many cases, the segment or lobe reexpands and the radiographic abnormalities resolve completely. However, these children are still at risk for developing reactivation tuberculosis later in life. In some cases, this segmental lesion resolves, but residual calcification of the parenchymal focus and regional lymph node occurs. Finally, bronchial obstruction may cause scarring and progressive contraction of the lobe or segment, which may be associated with cylindrical bronchiectasis and chronic pyogenic infection.
A rare but serious complication of tuberculosis in children occurs when the parenchymal focus enlarges and develops a large, caseous center. This progressive primary tuberculosis presents like bronchopneumonia, and may be accompanied by high fever, severe cough, dullness to percussion, rales, and decreased breath sounds. Liquefaction in the center may result in formation of a thin-walled cavity. Before the advent of antituberculosis chemotherapy, the mortality rate of this form of tuberculosis was 30% to 50%. With effective treatment, the prognosis is excellent for full recovery.
Pleural
Tuberculous pleural effusions, which can be local or general, originate from the discharge of bacilli into the pleural space from a subpleural pulmonary focus or caseated subpleural lymph node. Asymptomatic local pleural effusion is so frequent in childhood pulmonary tuberculosis that it is basically a component of the primary complex. Most large and clinically significant effusions occur months to years after the initial infection. Tuberculous pleural effusion is infrequent in children younger than 6 years and rare in those younger than 2 years. Effusions are usually unilateral, but they can be bilateral.
The clinical onset of tuberculous pleurisy is usually fairly sudden. It is characterized by low to high fever, shortness of breath, chest pain on deep inspiration, dullness to percussion, and diminished breath sounds on the affected side. The presentation is similar to that of pyogenic pleurisy. The fever and other symptoms may last for several weeks after the start of ultimately effective antituberculosis chemotherapy. Although corticosteroids may reduce the clinical symptoms, they have little effect on the ultimate outcome. The tuberculin skin test is positive in only 70% to 80% of cases. The prognosis is excellent, but radiographic resolution may take months. Scoliosis rarely complicates recovery of a long-standing effusion.
Cardiac
Tuberculous pericarditis occurs in only 0.4% of infected children. It arises from hematogenous dissemination or direct invasion from caseous lymph nodes in the subcarinal area. Pericardial fluid may be serofibrinous or hemorrhagic. However, tubercle bacilli rarely are found on direct smear of the fluid. Extensive fibrosis of the pericardial sac may lead to obliteration with development, usually years later, of constrictive pericarditis. The presenting systems are usually nonspecific: low-grade fever, poor appetite, failure to gain weight, and chest pain. A pericardial friction rub may be heard, or, if a large effusion already is present, distant heart sounds, tachycardia, and narrow pulse pressure may suggest the diagnosis. In the prechemotherapy era, half the patients died; now, with appropriate drugs and use of corticosteroid therapy to diminish the size of the effusion, the prognosis is excellent.
Disseminated (Miliary)
The lymphohematogenous spread of bacilli that accompanies the initial infection is usually asymptomatic. Although the clinical picture may be acute, more often it is indolent and prolonged, with high fevers accompanying the release of organisms into the bloodstream. Culture confirmation can be difficult. Bone marrow or liver biopsy with appropriate stains and cultures may be necessary and should be performed if the diagnosis is considered and other tests are unrevealing.
The most common clinically significant form of disseminated tuberculosis is miliary disease, which occurs when massive numbers of bacilli are released into the bloodstream, causing disease in at least two organs.12 This form of disease usually occurs within 2 to 6 months of the primary infection. The clinical manifestations are protean, depending on the number of organisms that disseminate and the focus of infection. Lesions are usually larger and more numerous in the lungs, spleen, liver, and bone marrow than in other organs. This form of tuberculosis is most common in infants and in malnourished or immunosuppressed patients. The onset of clinical disease is sometimes explosive, with the patient becoming gravely ill in several days. More often, the onset is insidious, the patient not being able to pinpoint the true time of initial symptoms. The most common signs include malaise, anorexia, weight loss, and low-grade fever. Within several weeks hepatosplenomegaly and generalized lymphadenopathy develop in about 50% of cases. About this time, the fever may become higher and more sustained, but the chest radiograph is usually normal and respiratory symptoms are few. Within several more days to weeks, the lungs become filled with tubercles, causing dyspnea, cough, rales, and wheezing. As pulmonary disease progresses, alveolar air block syndrome may result in frank respiratory distress, hypoxia, and pneumothorax or pneumomediastinum. Signs or symptoms of meningitis or peritonitis are found in 20% to 40% of patients with advanced disease. Severe headache in a patient with miliary tuberculosis usually indicates the presence of meningitis. Abdominal pain or tenderness is usually a sign of tuberculous peritonitis. Choroid tubercles occur in 13% to 87% of patients and are highly specific for miliary tuberculosis. Unfortunately, the tuberculin skin test is nonreactive in as many as 50% of patients with advanced disease.
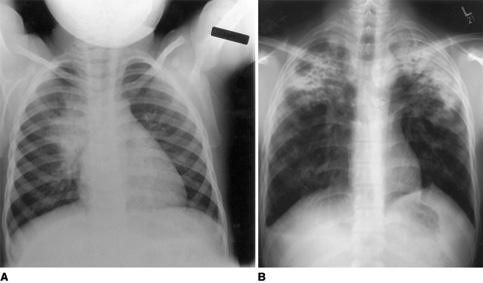
FIGURE 269-1. A: A chest radiograph from a child with early pulmonary tuberculosis demonstrating hilar adenopathy and perihilar infiltrate. B: An adolescent with severe bilateral upper lobe tuberculosis, with cavitation on the right side.
Central Nervous System
Central nervous system tuberculosis is the most serious complication in children and is fatal without effective treatment. This condition can arise from massive hematologic dissemination of organisms, but usually arises from the formation of a caseous lesion in the cerebral cortex or meninges that develops during the occult lymphohematogenous dissemination of the initial infection. This lesion, called a Rich focus, increases in size and discharges small numbers of tubercle bacilli into the subarachnoid space. The resulting exudate may infiltrate the cortical or meningeal blood vessels, producing inflammation, obstruction, and subsequent infarction of the cerebral cortex. This exudate also interferes with the normal flow of CSF in and out of the ventricular system at the level of the basal cisterns, leading to a communicating hydrocephalus. The combination of vasculitis, infarction, cerebral edema, and hydrocephalus results in severe damage that occurs gradually or rapidly. Abnormalities in electrolyte metabolism, especially hyponatremia caused by SIADH or salt wasting, also contribute to the pathophysiology.
Tuberculous meningitis complicates about 0.3% of untreated tuberculosis infections in children. This condition is extremely rare in infants younger than 3 months because pathologic events usually need this much time to develop. It is most common in children between 6 months and 4 years of age.
The clinical progression of tuberculous meningitis may be rapid or gradual. The signs and symptoms progress slowly over several weeks and can be divided into three stages. The first stage, which typically lasts 1 to 2 weeks, is characterized by nonspecific symptoms such as fever, headache, irritability, drowsiness, and malaise. Focal neurologic signs are absent, but infants may experience a stagnation or loss of developmental milestones. The second stage usually begins more abruptly. Lethargy, nuchal rigidity, Kernig and Brudzinski signs, seizures, hypertonia, vomiting, cranial nerve palsies relevant to basilar meningitis, and other focal neurologic signs are apparent. This clinical picture usually correlates with the development of hydrocephalus, increased intracranial pressure, and vasculitis.13 The third stage is marked by coma, hemiplegia or paraplegia, hypertension, decerebrate posturing, deterioration in vital signs, and, eventually, death. The prognosis of tuberculous meningitis correlates closely with the clinical stage of illness at the time treatment with antituberculosis chemotherapy and corticosteroids begins.14 The majority of patients in the first stage have an excellent outcome, whereas most patients diagnosed in the third stage who survive have permanent disabilities, including blindness, deafness, paraplegia, and mental retardation. It is imperative that antituberculosis chemotherapy be considered for any child who develops basilar meningitis and hydrocephalus or cerebral infarction with no other apparent etiology. The key to diagnosis is often identifying the adult from whom the child acquired M tuberculosis.
Another manifestation of central nervous system tuberculosis is the tuberculoma, which presents clinically as a brain tumor. Tuberculomas account for as many as 40% of brain tumors in children in some areas of the world, but they are rare in North America. These lesions, which occur most often in children younger than 10 years, are usually singular, but they may be multiple. The most common symptoms are headache, fever, and seizures. The paradoxical development of tuberculomas in patients with tuberculous meningitis while receiving effective chemotherapy has been recognized since the advent of CT. The cause and nature of these tuberculomas are poorly understood, but their development does not require a change in the therapeutic regimen. Whenever a child with tuberculous meningitis deteriorates or develops focal neurologic findings while on treatment, this phenomenon should be considered. Corticosteroids may help alleviate the occasionally severe clinical signs and symptoms. These lesions may be very slow to resolve clinically, persisting radiographically for months or years.
Lymph Node
Tuberculosis of the superficial lymph nodes is the most common form of extrapulmonary tuberculosis in children. Most cases occur within 6 to 9 months of the initial infection, although some cases appear years later. The tonsillar, anterior cervical, and submandibular nodes become involved secondary to extension of a primary lesion of the upper lung fields or abdomen.
In the early stages of infection, the lymph nodes usually enlarge gradually.15 The nodes are firm but not hard, discrete, and nontender. The nodes usually feel fixed to underlying or overlying tissue. Disease is most often unilateral, but bilateral involvement may occur. As infection progresses, multiple nodes are affected, resulting in a mass of matted nodes. Systemic signs and symptoms other than low-grade fever are usually absent. The chest X-ray is usually normal, although adenopathy in the chest may be apparent. Occasionally, the illness is more acute with rapid enlargement of cervical nodes, high fever, tenderness, and fluctuance. 
Skeletal
Skeletal tuberculosis results from lymphohematogenous seeding of tubercle bacilli during the initial infection. Bone infection also may originate as a result of direct extension from a regional lymph node or a neighboring infected bone. The time interval between infection and clinical disease can be as short as 1 month in cases of tuberculous dactylitis, or as long as 30 months or more for tuberculosis of the hip. The infection usually begins in the metaphysis. Granulation tissue and caseation destroy bone by direct infection and by pressure necrosis. Soft tissue abscess and extension of the infection through the epiphysis into the nearby joint often complicate the bony lesion.
Weight-bearing bones and joints are most commonly affected.16 The majority of cases of bone tuberculosis occur in the lower thoracic and upper lumbar vertebrae, causing tuberculosis of the spine or Pott disease. Involvement of two or more vertebrae is common; these vertebrae are usually contiguous, but there may be skip areas between lesions. Infection in the body of the vertebra leads to bony destruction and collapse. The infection may extend out from the bone, causing a paraspinal, psoas, or retropharyngeal abscess. The most frequent clinical signs and symptoms of tuberculous spondylitis in children are low-grade fever, irritability, and restlessness, especially at night; back pain; and abnormal positioning in gait or refusal to walk. Other sites of skeletal tuberculosis, in approximate order of frequency, are the knee, hip, elbow, and ankle. The tuberculin skin test is reactive in 80% to 90% of cases, and culture of joint fluid or bone biopsy usually yields the organism.
Tuberculous dactylitis is a form of bone tuberculosis that is peculiar to infants. Affected children develop distal endarteritis followed by painless swelling and cystic bone lesions in the hands.
Abdominal and Gastrointestinal
Tuberculous enteritis is caused by hematogenous dissemination of organisms in most cases. However, ingestion of unpasteurized cow’s milk laden with Mycobacterium bovis still causes this disease in many areas of the world. The jejunum and ileum near Peyer patches and the appendix are the most common sites of involvement. Mesenteric adenitis usually complicates this disease. Lymph nodes may cause intestinal obstruction or erode through the omentum to cause generalized peritonitis. This entity should be considered in any child with chronic gastrointestinal complaints and a reactive tuberculin skin test.
Genitourinary
Renal tuberculosis is rare in children, and the incubation period is several years or longer. Tubercle bacilli can be isolated from the urine in cases of miliary tuberculosis, even in the absence of renal disease. In true renal tuberculosis, small caseous tubercles develop in the renal parenchyma and release M tuberculosis into the tubules. Renal tuberculosis is often clinically silent in the early stages. The only signs may be sterile pyuria and microscopic hematuria. As the disease progresses, dysuria, flank, or abdominal pain and gross hematuria develop. Superinfection by other bacteria is frequent and may delay recognition of the underlying tuberculosis. Hydronephrosis or ureteral stricture may complicate the disease.
Tuberculosis of the genital tract is uncommon in both males and females before puberty. This condition usually originates from lymphohematogenous spread, but can complicate direct spread from the intestinal tract or bone. In adolescent girls, the fallopian tubes are most often involved, followed by the endometrium, the ovaries, and the cervix. The usual symptoms are low abdominal pain and dysmenorrhea or amenorrhea. Chronic infection usually leads to infertility.
Other Sites
Cutaneous tuberculosis, which was more common decades ago, arises as an extension of disease from the primary infection, from hematogenous dissemination, or from hypersensitivity to the bacilli. Skin lesions associated with the initial infection can be caused by direct inoculation of the skin through an abrasion, cut, or insect bite. Regional lymphadenitis is striking, but systemic symptoms are usually absent. The most common form of hypersensitivity lesion is erythema nodosum, which is characterized by large, painful, purple-brown, indurated nodules on the shins and forearms. Scrofuloderma occurs when a caseous lymph node ruptures to the outside and leaves an ulcer or sinus tract.
Ocular tuberculosis is very rare in children. This condition usually involves the conjunctiva or cornea and results from direct inoculation. 
Congenital
True congenital tuberculosis is exceedingly rare, with less than 400 cases reported. M tuberculosis can pass from the placenta to the fetus through the umbilical vein. The mothers of these infected infants frequently suffer from tuberculous pleural effusion, meningitis, or disseminated disease during pregnancy or soon afterward. Initial infection in the mother just before or during pregnancy is more likely to lead to congenital infection than previous infection. The bacilli in the lung usually remain dormant until after birth, when oxygenation and pulmonary circulation increase significantly. Congenital tuberculosis may also occur by aspiration or ingestion of infected amniotic fluid if a caseous placental lesion ruptures directly into the amniotic cavity.
Symptoms of true congenital tuberculosis may be present at birth, but more commonly begin in the second or third week of life. The most common signs and symptoms, in order of frequency, are respiratory distress, fever, hepatic or splenic enlargement, poor feeding, lethargy or irritability, lymphadenopathy, abdominal distention, failure to thrive, ear drainage, and skin lesions. Many infants have an abnormal chest radiograph, most often a miliary pattern. This clinical presentation in newborns is similar to that caused by bacterial sepsis and other congenital infections.
Tuberculosis and HIV Infection
In general, the clinical presentation of tuberculosis in children with HIV infection is similar to that in children without HIV infection.18 However, children with HIV infection more commonly have extrapulmonary tuberculosis (especially meningitis, tuberculoma, and abdominal disease), and pulmonary tuberculosis has a more aggressive picture, more often leading to substantial infiltrates or cavitation within the lung. Establishing the diagnosis of tuberculosis in an HIV-infected child can be difficult because the skin test is often negative, microbiologic confirmation of disease is difficult to achieve in many cases, and other opportunistic conditions can mimic tuberculosis. An aggressive evaluation for tuberculosis should be undertaken for any child with known HIV infection, or risk factors for HIV infection, who develops pulmonary disease or any unusual constellation of signs and symptoms.
 DIAGNOSIS
DIAGNOSIS
There are two primary ways in which a child with tuberculosis can be discovered. The first is when tuberculosis is considered part of the differential diagnosis of a symptomatic child. This passive discovery is usually the only means of diagnosis in resource-poor countries, and children often have advanced disease. The second is when children with tuberculosis in developed countries are discovered through contact investigations of adults who are believed to have infectious tuberculosis. In these cases, children usually have relatively asymptomatic disease that would have either progressed or escaped detection if the contact tracing had not occurred. The importance of the epidemiologic setting of the child in establishing the diagnosis of tuberculosis cannot be overemphasized. Often, the most important maneuver in determining whether the child has tuberculosis is testing the adults in close contact with the child to determine whether any adult has or recently has had infectious pulmonary tuberculosis.
General laboratory and other tests are usually unrevealing for children with tuberculosis. Screening tests such as a complete blood count and differential, erythrocyte sedimentation rate, and blood chemistries are usually normal. When considering a diagnosis of extrapulmonary tuberculosis, analysis of appropriate tissue or fluids often leads to establishing the correct diagnosis. In cases of tuberculous meningitis, the CSF leukocyte count usually ranges from 10 to 500 cells/mm3, but is occasionally higher. Polymorphonuclear leukocytes may be common initially, but in a majority of cases, lymphocytes are predominant. The CSF glucose level is typically less than 40 mg/dL, but rarely goes below 20 mg/dL. The protein level is elevated and may be markedly high (400–5000 mg/dL) secondary to hydrocephalus and spinal block. Although the lumbar CSF is grossly abnormal, ventricular CSF may have normal chemistries and cell counts because samples are obtained proximal to the site of obstruction.
In cases of pleural tuberculosis, the pleural fluid usually yields results indicative of a mild exudate: specific gravity is 1.012 to 1.025, the protein level is usually 2 to 4 g/dL, and the glucose may be low, although it is often in the low-normal range (20–40 mg/dL). There are typically several hundred to several thousand white blood cells/mm3, with an early predominance of polymorphonuclear cells followed by a high concentration of lymphocytes. Biopsy of the pleura may show evidence of granuloma formation and the organisms.
Tuberculin Skin Testing
A positive tuberculin skin test is the hallmark of infection with M tuberculosis.19 The definitive test is the Mantoux skin test technique, which in the United States involves intradermal injection of 0.1 mL of purified protein derivative containing five tuberculin units. The results are interpreted as the transverse diameter of induration present 48 to 72 hours after injection. A variety of host-related factors—including very young age, malnutrition, immunosuppression by disease or drugs, viral infection, measles vaccination, and overwhelming tuberculosis—can depress tuberculin reactivity in a child infected with M tuberculosis. Approximately 10% of immunocompetent children with tuberculosis disease do not react initially to a tuberculin skin test; however, most become reactive after several months of treatment, suggesting that the disease contributed to this anergy. Anergy with tuberculosis may be global or specific to tuberculin, so a positive control skin test with a negative tuberculin test never rules out tuberculosis disease. False-positive reactions to tuberculin skin tests can be caused by cross-sensitization to antigens of nontuberculous mycobacteria or, in some cases, previous immunization with bacille Calmette-Guérin (BCG) vaccine. No reliable method distinguishes tuberculin reactions caused by a BCG vaccination from those resulting from infection with M tuberculosis. However, many infants who receive a BCG vaccine never develop a positive tuberculin reaction. When a reaction does occur, the induration is usually less than 10 mm and the reaction often wanes after several years. One study of BCG cross-reaction in Native American children vaccinated at birth showed that all positive Mantoux reactions occurred within the first 6 months after vaccination. In general, a reactive area of 10 mm or more in a BCG-vaccinated child indicates infection with M tuberculosis and necessitates further diagnostic evaluation and treatment. A history of prior BCG vaccination is never a contraindication to tuberculin testing.
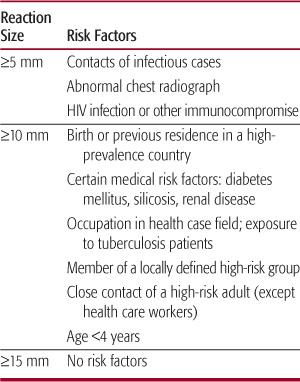
The interpretation of the tuberculin reaction should be influenced by the purpose for which the test was given and the consequences of false classification.20 Because there is always some overlap in reactions to the Mantoux test between groups of individuals with and without infection with M tuberculosis, false-positive and false-negative results always occur within a population. To try to minimize false results, reaction size limits for determining a positive result are made. Patients are then stratified by risk of infection (Table 269-1). For adults and children at the highest risk of having infection progress to disease, a reactive area of at least 5 mm is classified as a positive result. For other high-risk groups, including children younger than 4 years, a reactive area of at least 10 mm is considered positive. For all other low-risk persons, the cutoff point for a positive reaction is raised to 15 mm. The key to this scheme is obtaining an adequate history of possible risk factors for acquiring infection with M tuberculosis. Classifying children by this scheme depends on the willingness and ability of the clinician and family to create a thorough history for the child and for the adults who are in the child’s environment. In general, tuberculin skin testing of low-risk children yields few positive results, and in low prevalence populations the majority of these positive results will be false positive.
Interferon Release Assays
Advances in molecular biology and genomics have led to alternatives to the TST.21 Two new in vitro tests have been developed. Lymphocytes in whole blood are stimulated with M tuberculosis antigens, and the release of interferon-γ is measured. Both tests use two proteins—early secreted antigen target-6 and culture filtrate protein—that are found on M tuberculosis and only a few fairly rare species of nontuberculous mycobacteria, but not on the bacilli Calmette-Guérin (BCG) or the Mycobacterium avium complex. The first test is QuantiFERON-TB (QFT; Cellestis, Carnegie, Australia), which measures whole blood IFN production. The second format is the enzyme-linked immunospot (ELISPOT) (T-SPOT.TB; Oxford Immunotec, Oxford), which measures the number of mononuclear cells that produce INF.
In many clinical situations, these tests have a higher specificity than the TST, better correlation with surrogate measures of recent exposure to M tuberculosis in low incidence settings and less cross-reactivity than the TST caused by previous BCG vaccination. Two clear advantages of the IFN release assays are the need for only one patient encounter (two with the TST) and the lack of possible boosting of the result because the patient is not exposed to any biologic material. 
Stains and Cultures
The most important laboratory tests for the diagnosis of tuberculosis are the acid-fast stain and mycobacterial culture. The best culture specimen for pulmonary tuberculosis in a child has been the early morning gastric aspirate obtained before the child has risen and before peristalsis has emptied the stomach of the pooled secretions that were swallowed overnight. In general, acquisition of these samples has required hospitalization. Unfortunately, even under optimal conditions, three gastric aspirates yield M tuberculosis in less than 50% of cases. Therefore, negative cultures never exclude the diagnosis of tuberculosis in a child. The culture yield from bronchoscopy in children with tuberculosis is usually less than the yield from properly obtained gastric samples. More recent studies from South Africa have demonstrated a culture yield from out-patient induced sputum (using warm nebulized saline and a suction catheter to capture the mucus) equal to that for inpatient gastric aspirates in children with extensive pulmonary disease.
Table 269-1 Amount of Induration That Defines a Positive Mantoux Tuberculosis Skin Test
Fortunately, the need for culture confirmation in children with tuberculosis is not always necessary. If a child has a positive tuberculin skin test, clinical or radiographic findings suggestive of tuberculosis, and known contact with an adult case of tuberculosis, the child should be treated for tuberculosis disease. The drug susceptibility test results from the adult case can be used to determine the best therapeutic regimen for the child. Cultures always should be obtained from a child with suspected tuberculosis when the source case is not known, or when the source case has a drug-resistant isolate.
Unfortunately, acid-fast stain of various fluids and tissues from children with tuberculosis disease is often unrevealing. Acid-fast stain of gastric samples is positive in less than 10% of cases, and staining and culture of other infected material is positive in less than 25% to 50% of cases.
Nucleic Acid Amplification
The main form of nucleic acid amplification studied in children with tuberculosis is the polymerase chain reaction (PCR), which uses specific DNA sequences as markers for microorganisms. Various PCR techniques have a sensitivity and specificity of more than 90%, as compared with sputum culture, for detecting pulmonary tuberculosis in adults. However, in children, the sensitivity of PCR has varied from 25% to 83%, and specificity has varied from 80% to 100% when compared to clinical diagnosis. A negative PCR result never eliminates tuberculosis as a diagnostic possibility. The major use of PCR is in evaluating children with significant pulmonary disease when the diagnosis is not established readily by clinical or epidemiologic grounds. PCR may be particularly helpful in evaluating immunocompromised children with pulmonary disease, or in children with extrapulmonary disease.
 TREATMENT
TREATMENT
Mycobacteria replicate slowly and remain dormant in the body for prolonged periods. The treatment of tuberculosis is affected by the presence of naturally occurring drug-resistant organisms in large bacterial populations, even before chemotherapy is initiated. This drug resistance is caused by mutation at one of several chromosomal loci. Although a population as a whole may be considered drug susceptible, a subpopulation of drug-resistant organisms occurs at fairly predictable frequencies within the main population. A cavity containing 109 bacilli will have thousands of drug-resistant organisms, whereas a caseous lesion with a much smaller population contains but few resistant organisms.
These microbiologic characteristics of M tuberculosis explain why single antimicrobial drugs cannot cure tuberculosis disease in adults. The major biologic determinant of the success of antituberculosis therapy is the size of the bacterial population within the host. For patients with a large population of bacilli, such as adults with cavities or extensive infiltrates, many drug-resistant organisms are present initially, and at least two antituberculosis drugs must be given. Conversely, for patients with infection but no disease, the bacterial population is small, drug-resistant organisms are rare or nonexistent, and a single drug, such as isoniazid, can be given. Children with pulmonary tuberculosis and patients with extrapulmonary tuberculosis have medium-size populations in which significant numbers of drug-resistant organisms may or may not be present. In general, these patients are treated with at least two, and usually three or four, drugs.
Drugs for Tuberculosis
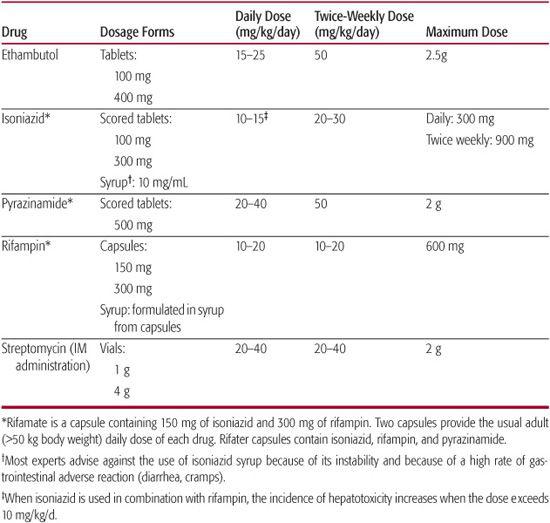
Table 269-2 details the first-line antituberculosis drugs used to treat tuberculosis in children.
Isoniazid
Isoniazid (INH), a synthetically produced drug, is the most potent and valuable single drug in the treatment of tuberculosis. An oral dose attains a plasma concentration 20 to 80 times the usual level required to inhibit the growth of tubercle bacilli (0.02–0.05 μg/mL) within several hours, with high concentrations persisting for 6 to 8 hours in plasma and sputum. INH penetrates readily into the CSF, even in the absence of inflammation, and into caseous tissue.
The principal side effects of INH are peripheral neuritis and hepatitis. Peripheral neuritis results from competitive inhibition of pyridoxine metabolism. This is more likely to occur at higher dosages of INH (> 10 mg/kg/d) in alcoholics and people who are poorly nourished. This is rarely a problem in children, although precautions must be taken during adolescence, for breast-feeding babies, during pregnancy, or when the total daily dose of INH exceeds 300 mg. Pyridoxine (10 mg for each 100 mg of INH) should be given daily when indicated.
Hepatotoxicity is a problem in patients older than 35 years. In children, hepatitis is rare and usually mild. Slight elevation of serum liver enzymes occurs in 1% to 5% of children taking INH, but symptomatic hepatitis is very rare. Concomitant use of rifampin or phenytoin increases the likelihood of hepatitis, as do INH dosage regimens in excess of 15 mg/kg/d.
Children who are taking INH need not have serum liver enzyme testing unless they have a previous history of liver disease, predisposition to the development of liver disease, or are taking other liver toxic drugs. A careful symptom review should be performed monthly, with warnings to report such symptoms as nausea, loss of appetite, or right upper-quadrant pain promptly.
Rifampin
Rifampin is a semisynthetic drug that has wide antimicrobial activity against bacteria and mycobacteria. It is absorbed readily from the gastrointestinal tract after oral administration, with peak concentrations of 6 to 32 μg/mL (MIC for M tuberculosis 0.5 μg/mL) occurring in 3 hours. Rifampin readily diffuses to most tissues and body fluids; CSF levels are low but adequate for treatment. It is excreted primarily through the biliary tract and kidneys.
Table 269-2 First-Line Drugs Used to Treat Tuberculosis Infection and Disease in Children
Rifampin is relatively nontoxic; the principal side effect is hepatitis, which occurs with a frequency of < 1%. Hepatitis seems to be more common in patients who are treated with the combination of rifampin and INH. Gastrointestinal disturbances, rashes, reversible leukopenia, thrombocytopenia, and elevation of blood urea nitrogen have been reported. Rifampin interacts with many drugs, including dicumarol, corticosteroids, antifungal agents, and many anti-HIV agents. Rifampin may chemically interfere with birth control pills, making them ineffective. Administration of the drug may also impart an orange-red color to feces, urine, sputum, saliva, tears, and sweat. The suggested dosage is 10 to 20 mg/kg/d (maximum, 600 mg). A liquid preparation is not commercially available, but can be prepared in community pharmacies.
Pyrazinamide
Pyrazinamide (PZA) is a bactericidal drug that attains a therapeutic concentration in the CSF and in macrophages. It is recommended as the third drug of a three- or four-drug regimen, particularly for the first 2 months of therapy. In doses of 20 to 40 mg/kg/d (adult dose 2 g/d), it is well tolerated by children. Adverse reactions are rare in children, but may include hepatitis, joint pain, and itching with or without a rash.
Ethambutol
Ethambutol is an odorless water-soluble compound rapidly absorbed from the gastrointestinal tract and excreted in the urine, mainly with its form unchanged. It is bacteriostatic at the usual dose of 20 mg/kg/d. The only important toxic effect is a retrobulbar neuritis that infrequently results in loss of visual acuity, defects in visual fields, and inability to distinguish between red and green; the visual changes are usually reversible. This side effect should be monitored by monthly studies of visual acuity and visual fields and tests for green color vision when possible. The inability to monitor for this complication has limited the use of this agent in young children. However, at doses of 20 mg/kg/d, it can be safely administered to children of all ages.23 Ethambutol is used as the fourth drug in a multidrug regimen, and its major purpose is to prevent emergence of resistance to other drugs.
Streptomycin
Streptomycin was the first effective antituberculosis drug. It is given intramuscularly and is rapidly absorbed into the bloodstream, reaching peak levels that are 50 to 100 times more than the MIC of 0.2 μg/mL. It diffuses readily into the pleural fluid, but does not diffuse into the CSF unless the meninges are inflamed. Streptomycin is excreted mainly in the urine, with 80% recovery within 24 hours. The principal toxic effect is eighth nerve damage, mainly of the vestibular branch, resulting in vertigo and ataxia that is usually permanent. Hearing loss is less common and usually affects the high-frequency range before affecting the lower frequencies. At current dosage schedules, hearing defects are rare in children. Streptomycin is a second-line drug in most developed countries, used mostly in cases of drug-resistant tuberculosis.
Corticosteroids
These drugs are controversial in the management of tuberculosis. They can be used only if effective antituberculosis therapy is in place. They are useful when the host inflammatory response to M tuberculosis contributes to tissue damage. Generally accepted indications are for the management of tuberculous meningitis, tuberculous pleural effusion, pericarditis, and endobronchial disease. Prednisone at 2 mg/kg/d is used commonly for 4 to 6 weeks, then weaned slowly.
Other Drugs
The emergence of multidrug-resistant M tuberculosis strains occasionally requires the use of secondary drugs. Ethionamide, cycloserine, capreomycin, amikacin, rifabutin, and fluoroquinolones have been used, but an expert in tuberculosis always should be consulted when their use is contemplated in a child.
Treatment of Exposure and Infection
In the United States, children exposed to potentially infectious adults with pulmonary tuberculosis should be started on treatment with isoniazed if the child is younger than 5 years or has other risk factors for the rapid development of tuberculosis disease. Failure to do so may result in the development of severe tuberculosis even before the tuberculin skin test becomes reactive; the “incubation” of disease may be shorter than that for the skin test. The child is treated for a minimum of 3 months after contact with the infectious case is broken. After 3 months, the tuberculin skin test is repeated. If the second test is positive, infection is documented and isoniazid should be continued for a total of 9 months; if the second skin test is negative, the treatment can be stopped.
Two circumstances of exposure deserve special attention. A difficult situation arises when exposed children are anergic because of HIV infection or other immunocompromise. These children are particularly vulnerable to rapid progression of tuberculosis, and it may not be possible to tell whether infection has occurred. In general, these children should be treated as if they have tuberculosis infection. The second situation is potential exposure of a newborn to a mother or other adult with possible pulmonary tuberculosis. In general, this exposure should be treated the same as for an older infant. The neonate should be started on isoniazid and continued on it until tuberculosis disease in the adult can be ruled out, or for 3 months after the person with tuberculosis is no longer contagious.
The treatment of children infected with M tuberculosis before they have developed disease is a mainstay of modern tuberculosis control.20 Many large, well-documented studies have shown that isoniazid is extremely effective in preventing the development of tuberculosis disease in infected children. Because isoniazid is so safe in this age group, any child or adolescent with a “positive” tuberculin skin test or interferon-gamma release assay result and no evidence of tuberculosis disease should receive treatment. In most cases, treatment is 9 months of isoniazid. Isoniazid is usually given every day by self-supervision, but can be administered twice weekly under the direct observation of a health care worker in cases of high-risk infection, particularly if an adult with active tuberculosis who is also being treated twice a week is present in the home. The optimal length of isoniazid therapy has been debated for 40 years. The summary opinion of experts is that 9 months of therapy is the optimal length of treatment for children with tuberculosis infection.
If a child is exposed to or infected with an isoniazid-resistant but rifampin-susceptible strain of M tuberculosis, rifampin should be given for 6 months. If the infecting strain is resistant to both isoniazid and rifampin, usually two other drugs are used, but an expert in tuberculosis should be consulted for this situation.
Treatment of Disease
Over the past three decades, a large number of trials of antituberculosis therapy for children with drug-susceptible pulmonary tuberculosis have demonstrated that the optimal regimen is 6 months’ duration, starting with at least three antituberculosis medications, usually isoniazid, rifampin, and pyrazinamide.24 Isoniazid and rifampin are continued for the entire 6 months, whereas pyrazinamide is used only for the first 2 months of therapy. Medications are usually given every day for the first 2 weeks to 2 months of therapy. After this time, medications can be given safely and effectively twice or thrice weekly under the observation of a health care worker. In all the reported trials for these regimens, the overall success rate for therapy was greater than 98%, and the incidence of clinically significant adverse reactions was less than 2%. If the child is at risk for being infected with isoniazid-resistant tuberculosis because of previous treatment of the adult source case, or because the child has lived in an area of the world where resistance rates are high, most experts would add a fourth drug, usually ethambutol, to the initial regimen, until the exact drug susceptibility of either the child’s isolate or the adult source case’s isolate can be established.
Controlled clinical trials for treating various forms of extrapulmonary tuberculosis are almost nonexistent.25 Extrapulmonary tuberculosis is usually caused by fairly small numbers of mycobacteria. Most non–life-threatening forms of extrapulmonary tuberculosis respond well to a 6-month treatment regimen using three or four drugs in the initial phase, similar to that used for pulmonary tuberculosis. One exception may be bone and joint tuberculosis, which is associated with a higher failure rate when only 6 months of chemotherapy is used, especially if surgical intervention has not been performed. Some experts recommend at least 9 to 12 months of therapy for bone and joint tuberculosis. Tuberculous meningitis has not usually been included in trials of extrapulmonary tuberculosis because of its serious nature and low incidence. Several more recent trials suggest that 6 to 9 months of therapy is effective if isoniazid, rifampin, and pyrazinamide are administered during the initial phase of treatment. The official recommendation of the American Academy of Pediatrics for tuberculous meningitis is 9 to 12 months of therapy that includes at least isoniazid and rifampin and usually one or two other drugs in the initial phase of treatment. Most experts add a fourth drug at the beginning of therapy to protect against initial drug resistance.
In general, the treatment of tuberculosis in HIV-infected children is the same as it is in children without HIV infection.26 Although some experts previously recommended lengthening the duration of therapy to 9 to 12 months in HIV-infected children, many trials have shown that adults with HIV infection and tuberculosis can be treated for the same length of time as adults without HIV infection who have tuberculosis.
The incidence of drug-resistant tuberculosis is increasing in many areas of the world. In the United States, approximately 10% of isolates of M tuberculosis are resistant to at least one drug. Many countries in Latin America and Asia routinely report drug resistance rates of 20% to 30%. Rates of drug resistance are unknown in many African countries. Patterns of drug resistance among children tend to mirror those found in adults in the same population. For children in the United States, certain epidemiologic factors, such as being immigrants from Asia or Latin America, or a history of previous antituberculosis treatment in the adult source, correlate with drug resistance. Therapy for drug-resistant tuberculosis is successful only when two bactericidal drugs to which the infecting strain of M tuberculosis is susceptible are given. When a child has a possible drug-resistant tuberculosis disease, at least three, and usually four or five, drugs should be administered initially, until the susceptibility pattern is determined and a more specific regimen can be designed. The specific treatment plan must be individualized for each patient, but durations of therapy of 12 to 18 months are not uncommon.
Activity does not need to be restricted in children with tuberculosis unless the child develops respiratory embarrassment or immobilization is needed for treatment, as in some cases of vertebral tuberculosis. Adequate nutrition is important, although reestablishment of weight gain may take several months. The major problem with treating tuberculosis in children and adults is nonadherence with therapy. Suspected cases of tuberculosis must be reported to the local health department so that it can compile accurate statistics, perform necessary contact investigations, and assist both patients and health care providers in overcoming barriers to adherence with therapy. In general, patients with tuberculosis disease should be treated with directly observed therapy, employing the help of a third party, such as a health department worker who observes the child and family during the administration of medication.
In general, children undergoing treatment for tuberculosis infection or disease should be seen every 4 to 6 weeks to monitor adherence, to observe for adverse reactions to medications, and to follow improvement in clinical course. Routine biochemical monitoring for adverse reactions is not necessary in asymptomatic children. Radiographic changes with intrathoracic tuberculosis occur slowly, and frequent chest radiographic monitoring is not necessary. A common practice for treating pulmonary tuberculosis is to obtain a chest radiograph at diagnosis and several months after the initiation of therapy to ensure that no unusual changes have occurred. Children with tuberculosis infection do not need a repeat chest radiograph.
 PREVENTION
PREVENTION
The only available vaccine against tuberculosis is BCG, which employs live attenuated bacilli. The BCG vaccines are extremely safe in immunocompetent hosts. BCG vaccination given during infancy has little effect on the ultimate incidence of tuberculosis among adults in a population. However, many experts believe that BCG vaccines are more effective in preventing disseminated tuberculosis among infants and young children. Retrospective studies from Europe and Asia yielded estimates of the protective effect of BCG in young children of 60% to 80%, and the effect is particularly strong for tuberculous meningitis and severe forms of disease. Local ulceration and regional suppurative lymphadenitis occur in 0.1% to 1% of vaccines. These lesions usually resolve spontaneously, but occasionally require chemotherapy with either isoniazid or erythromycin. Rarely, surgical incision of the suppurative draining node is necessary, but this should be avoided rather than encouraged. Systemic complaints such as fever, convulsions, and irritability are extraordinarily rare after BCG vaccination. Rare children with undiagnosed immunocompromising conditions (eg, severe combined immunodeficiency) develop systemic infection after neonatal BCG vaccination.
BCG vaccination works well in some situations, but poorly in others. Its only recommended use in the United States is for children who will invariably be exposed to adults with multidrug-resistant tuberculosis due to family and other epidemiologic factors.
 PROGNOSIS
PROGNOSIS
The prognosis of tuberculosis in infants, children, and adolescents is excellent with early recognition and effective chemotherapy. In most children with pulmonary tuberculosis, the disease completely resolves and, ultimately, radiographic findings are normal. The prognosis for bone and joint tuberculosis and for tuberculous meningitis depends on the stage of disease at the time antituberculosis medications are started. With all forms of extrapulmonary tuberculosis, the major problems are usually delayed recognition of the cause of disease and delayed initiation of treatment.
A resurgence of tuberculosis infection and disease is occurring among children in many regions of the world. As long as the conditions that promote tuberculosis, such as poverty, poor access to health care, overcrowding, and now HIV infection continue, this upward trend is likely to be sustained.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


