PREPARING CHILDREN AND INFANTS FOR TRAVEL
 Travel Plans
Travel Plans
Parents and care providers should be advised that travel with children and infants is much more enjoyable when the number of journeys in a single trip is limited; travel time is kept relatively short; and travel delays are anticipated. Planning for delays and other problems should include bringing new or favorite toys or games for distraction, and carrying extra food and drink, changes of clothing, and fever medications.
Medical Care During Travel
It is useful to obtain the names and addresses of local health care providers at the family’s destination. This is available from travel medicine practitioners or from the membership directory of the International Society of Travel Medicine. The International Association for Medical Assistance to Travelers website (www.iamat.org) is another useful resource with a worldwide directory of providers proficient in English. Travel insurance is highly encouraged. Insurance providers not only cover medical care at the destination, but provide 24-hour help lines with information regarding English-speaking physicians and hospitals, and can arrange and pay for evacuation to a medical facility that provides necessary treatment if not available locally. In emergencies, parents and caretakers should take their children to the largest medical facility in the area, which is more likely to have a pediatric unit and trauma services.
Trauma
Trauma is the most common cause of morbidity and mortality in traveling children. Parents should rent larger, safer vehicles, and use car seats whenever possible. However, in many developing countries, car seats are not available, so caretakers may need to travel with their own. Taxis often do not have seatbelts, so it may be necessary to request taxis with seatbelts by calling in advance.
Air Travel
Healthy term infants can travel by commercial pressurized airplane. Children at higher risk during air travel may include premature infants and those with chronic cardiac or pulmonary disease, so appropriate counseling with their specialist is indicated. Many parents request advice regarding sedation of their child during travel. While this is not recommended, the most widely used agent is diphenhydramine. It is advisable to try a test dose prior to travel as idiosyncratic reactions, and overdosing can lead to an anticholinergic syndrome or a paradoxical stimulating effect.
Ear Pain
Children and infants often have pain during ascent and descent of commercial airplanes due to changes in middle ear pressure causing retraction or protrusion of the tympanic membrane. Methods said to alleviate or minimize ear pain during these times include chewing, swallowing, nursing, and bottle feeding.
Motion Sickness
Almost 60% of children will experience motion sickness during travel. While older children have symptoms similar to those in adults (such as nausea, epigastric discomfort, headache, general discomfort), children younger than 5 years may have gait abnormalities as the predominant symptom. Nonpharmacologic strategies include eating a light meal at least 3 hours before travel; avoiding dairy products and foods high in calories, protein, and sodium before travel; sitting in the middle of the back seat or in the front seat if age-appropriate; focusing on a stable object or the horizon; avoiding reading or other visual stimuli; eye closure; fresh air; and limiting excessive head movement. Pharmacologic intervention has not been well studied in children, but if necessary, antihistamines such as diphenhydramine is recommended for children younger than 12 and scopolamine is acceptable for children older than 12 years. These measures, however, are not evidence-based.
High Altitude
Acute mountain sickness is as common in children as in adults, but it may go unrecognized due to its subtle presentation, such as unexplained fussiness or change in appetite and sleep patterns. High-altitude pulmonary edema (HAPE) is seen in children traveling to high altitudes; it also occurs in children who live at high altitude, descend for an extended period, and return to altitude. Mild symptoms of altitude sickness can be treated with rest and hydration, or analgesics such as ibuprofen or acetaminophen. High-altitude sickness is milder and resolves much more quickly in children compared to adults, so prophylaxis is usually not required. Acetazolamide has not been studied in children for acute mountain sickness, but it is safe in this age group and has been used for both prophylaxis and treatment. The pediatric dose is 5 mg/kg/d (125 mg maximum) divided twice daily, starting 1 day before ascent, and continued for 2 days at high altitude.
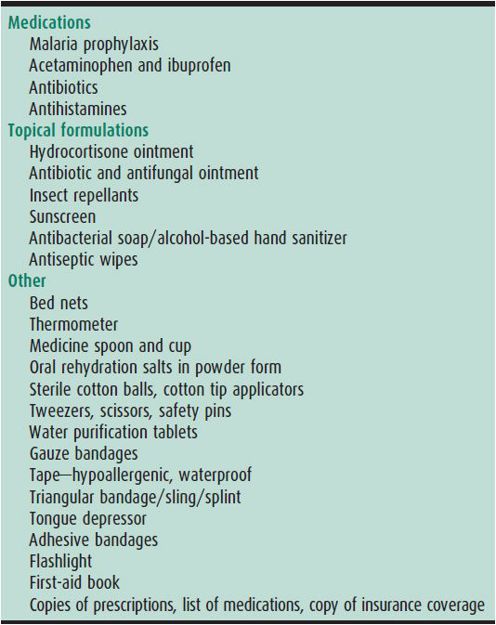
Medications/First-Aid Kit
A small medical kit is useful when traveling. Included in this kit should be medications for illnesses that the child experiences at home, trip-specific items, and the usual first-aid kit items (Table 45–2). Medications should be purchased prior to travel, as those obtained at some destinations may be of poor quality or contain toxic substances.
Table 45–2. First-aid kit for international travel.
Behrens RH, Carroll B. Travel trends and patterns of travel-associated morbidity. Infectious disease clinics of North America. 2012 Sep;26(3):791–802 [PMID: 22963784].
Dhillon S: Environmental hazards, hot, cold, altitude, and sun. Infectious disease clinics of North America. 2012 Sep;26(3):707–723 [PMID: 22963779].
Fhogartaigh CN, Sanford C, Behrens RH: Preparing young travellers for low resource destinations. Br Med J 2012;345:e7179 [PMID: 23131670].
Neumann K: Pediatric travel medicine: where we are and where we hope to go. J Travel Med 2012;19(3):137–139 [PMID: 22530818].
Schwartz BS, Larocque RC, Ryan ET: In the clinic. Travel medicine. Ann Intern Med 2012;156(11) [PMID: 22665823].
Stauffer W, Christenson JC, Fischer PR: Preparing children for international travel. Travel Med Infect Dis 2008;6(3):101–113 [PMID: 18486064].
Rexhaj E et al: Reproducibility of acute mountain sickness in children and adults: a prospective study. Pediatrics 2011;127(6):1445–1448 [PMID: 21536612].
VACCINATIONS—ROUTINE CHILDHOOD VACCINES MODIFIED FOR TRAVEL
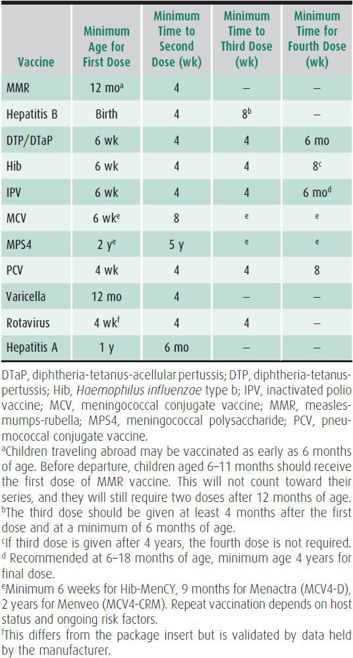
Many vaccine-preventable diseases remain prevalent in developing countries, and outbreaks still occur in areas where these diseases are considered rare. The schedule for some vaccines may be accelerated for travel, and some vaccines can be given earlier than the recommended age. Vaccination pertaining to children traveling follows the routine vaccination schedule as outlined in Chapter 10. The recommended intervals balance the high-risk age for disease with infant immunologic responses. The recommended minimum interval between doses is listed in Table 45–3. Barriers to some early immunizations are antibody from the mother interfering with an infant’s ability to mount an antibody response, particularly to live vaccines, and the lack of a T-cell–dependent immune response to certain immunogens in those younger than 2 years. For children traveling to highly endemic areas, however, it is preferable to vaccinate prior to the recommended age despite the possible need for repeat vaccination at a later date. Minor febrile illnesses are not a contraindication to routine or travel vaccines and should not lead to their postponement. Live vaccines should be given together or separated by 30 days or more.
Table 45–3. Accelerated vaccinations.
Diphtheria-Tetanus-Acellular Pertussis Vaccine
Immunization is recommended prior to travel to developing countries because of the greater risk of disease from diphtheria, tetanus, and pertussis. Tetanus risk is high in several areas of the developing world where fecal contamination of soil is extensive. Infants should receive their first diphtheria-tetanus-acellular pertussis (DTaP) at 6 weeks of age for an adequate immune response, with a 4-week interval between the subsequent two doses. Adequate protection is achieved after the third dose. The fourth dose may be given 6–12 months after the third dose provided that the child is 12 months of age or older. Tdap is licensed for children at least 11 years of age. Adolescents and adult caretakers, who are prominent vectors in the spread of pertussis to young children, should receive a single Tdap booster. If more than 5 years has elapsed since the last dose, a booster should be considered for children and adolescents to minimize tetanus risk. Tdap is preferred to Td in children older than 11 years if they have not received Tdap previously.
Haemophilus influenzae Type b Vaccine
The indications for vaccination of Haemophilus influenzae type b (Hib) in children traveling are the same as for the US residents. If previously unvaccinated, infants younger than 15 months should receive at least two doses prior to travel. An accelerated schedule can start at a minimum of 6 weeks of age, with a 4-week interval between the first, second, and third doses, and at least 8 weeks between third and fourth doses.
Hepatitis A Vaccine
Hepatitis A is one of the most common vaccine-preventable illnesses globally, and vaccination should be provided prior to travel to developing countries. In many developing countries, more than 90% of local children have hepatitis A antibodies by 6 years of age. The disease is much less common in children from the developed world, so they are likely to be susceptible when traveling to high-risk areas. Although two doses of the vaccine are recommended 6–12 months apart, a single dose will provide protection during the trip if given at least 2 weeks prior to departure. The earliest age of administration is 1 year in the United States. Immune globulin for hepatitis A (0.02 mL/kg IM) can be given for protection of children younger than 1 year, or if travel will occur within 2 weeks after vaccination. This interferes with MMR and varicella vaccination, so these vaccines should be given 2 weeks prior to immunoglobulin.
Hepatitis B Vaccine
Areas of high endemicity for hepatitis B include most of Asia, the Middle East, Africa, and the Amazon Basin. Unimmunized children are at risk if they receive blood transfusions that have not been screened for HBV surface antigen (HBsAg) or are exposed to unsterilized medical or dental equipment. Children traveling to developing countries should be vaccinated before departure. An accelerated schedule is possible, with the second dose given with a minimum 4-week interval, and the third dose given at least 8 weeks after the second dose. The third dose should not be given before 24 weeks of age.
Influenza Vaccine
Children are at high risk of respiratory infection during travel. The influenza vaccine is recommended for travel during the influenza season, which is between September and March in the Northern Hemisphere, and between April and August in the Southern Hemisphere, and year round in the tropics. The vaccine available in the United States may not protect against new strains circulating in the Southern Hemisphere. Influenza vaccine is recommended for children at least 6 months of age; those younger than 9 years will need two doses of vaccine administered at least 4 weeks apart if they have never previously received the vaccine containing the 2009 H1N1 antigen contained in all influenza vaccines 2010–2011 and beyond. The current vaccines available are: the trivalent or quadrivalent inactivated vaccine (IIV) given intramuscularly, and the quadrivalent live attenuated vaccine (LAIV) given intranasally. It is preferable to be vaccinated at least 2 weeks prior to departure. The annual seasonal influenza vaccine may not be routinely available in the United States from the late spring to early fall, when it may be needed for travelers but may be available at some travel clinics. Revaccination is not recommended for those who will be traveling during April through September and were vaccinated the preceding fall.
Measles-Mumps-Rubella (MMR) Vaccine
While measles is no longer endemic in the United States, it is still prevalent in many parts of the world, including Europe. Children as young as 6 months of age traveling outside the United States are recommended to receive the vaccine, but any doses given prior to 12 months do not count toward an adequate two-dose series, as maternal antibodies may interfere with the immune response. These infants will still require one dose of measles-mumps-rubella (MMR) at 12–15 months of age and a second dose at 4–6 years of age. The second dose is to protect those individuals (~5%) who did not respond the first time. If an accelerated schedule is required, two doses must be separated by minimum of 4 weeks.
Meningococcal Vaccine
The highest risk for meningococcal disease is for travelers to the meningitis belt of Africa (sub-Saharan region) especially during the dry season, and travelers on the Hajj or Umrah pilgrimage to Mecca. Notably meningococcal disease is decreasing in this region due to vaccination against type A. There are two meningococcal vaccines available. The conjugated quadrivalent (MCV4) vaccine is approved for use in persons aged 9–23 months (two doses 8 weeks apart) and 2–55 years (one dose). This vaccine is recommended for those who live or travel to areas with high rates of meningitis. It must be given at least 10 days before international travel. The quadrivalent meningococcal polysaccharide vaccine (MPSV4) is licensed for persons 2 years or older. Both types of vaccines protect against serotypes A, C, Y, and W-135. The duration of protection is 3 years in children and 5 years in adults, and boosters are indicated for ongoing exposure and at risk hosts. Hib-MenCY-TT is the approved vaccination for the age group 6 weeks to 9 months; as it does not provide protection against serotypes A or Y, a previously vaccinated infant reaching the age of 9 months and travelling to endemic areas should be revaccinated with MCV4. MPSV4 should be used for persons older than 56 years. Vaccination can be considered in children younger than 9 months with risk factors and/or traveling to an endemic area. Meningococcal vaccination is required by the Saudi Arabian government for pilgrims undertaking the Hajj or Umra pilgrimage to Mecca and Medina, because of the international outbreak of Neisseria meningitidis A in 1987 and W-135 in 2000 and 2001. Further information concerning geographic areas recommended for meningococcal vaccination can be obtained from http://www.cdc.gov/travel.
Recently, a new combined Hib and N meningitidis serogroup C conjugate vaccine has been licensed down to 6 weeks of age, but this should not be used for children travelling to the meningitis belt or the Hajj, as serogroup A is the predominant organism in these regions.
Pneumococcal Vaccine
Streptococcus pneumoniae infection is prevalent worldwide, and children younger than 2 years have the highest rates of disease. The 13-valent pneumococcal conjugate vaccine (PCV 13) is recommended for routine use in children aged 5 years or younger. In addition, the pneumococcal polysaccharide (PPSV23) is recommended for children and adults aged 2 years or older who have certain underlying medical conditions, and for all adults aged 65 years or older. For children aged 5 years or younger who have completed the PCV 7 series, a single additional dose of PCV 13 is recommended. The minimal interval is 4 weeks between the first three doses, and 8 weeks between the third and fourth dose.
Polio Vaccine
Transmission of wild-type polio still occurs in regions of Asia and Africa, and vaccine-derived polio is transmitted in other regions. Adequate immunization with inactivated polio vaccine (IPV) should be administered prior to travel to developing countries. The minimum age of administration is 6 weeks of age for IPV. The recommended interval between the first and second dose is 4 weeks as well as between the second and third dose, and 4 weeks between subsequent doses. One additional lifetime dose (a fifth dose) of the IPV should be given to caretakers who are traveling to areas with recent circulating polio.
Rotavirus Vaccine
Rotavirus is the most common cause of severe gastroenteritis in infants and young children worldwide, and vaccination with the complete series is recommended prior to travel if age-appropriate. The minimum and maximum age for the first dose is 4 weeks and 14 weeks, 6 days respectively. There is insufficient data on the safety in older infants. The minimum interval between doses is 4 weeks.
Cramer JP, Wilder-Smith A: Meningococcal disease in travelers: update on vaccine options. Curr Opin Infect Dis 2012 Oct;25(5):507–517 [PMID: 22907278].
Greenwood CS, Greenwood NP, Fischer PR: Immunization issues in pediatric travelers. Expert Rev Vaccines 2008 Jul;7(5):651–661 [PMID: 18564019].
Hill DR et al: The practice of travel medicine: Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006 Dec 15;43(12):1499–1539 [PMID: 17109284].
Wilder-Smith A: Meningococcal vaccines: a neglected topic in travel medicine? Expert Rev Vaccines 2009 Oct;8(10):1343–1350 [PMID: 19803757].
VACCINATIONS—TRAVEL-SPECIFIC
Japanese Encephalitis Vaccine
Japanese encephalitis (JE) is caused by a flavivirus transmitted by the night-biting Culex mosquito. The risk of contracting severe JE is low, especially for travelers who will have a brief stay in an endemic area, as the infection rate in Culex mosquitoes is 3% or lower, and only 1 in 200 infections with JE leads to neuroinvasive disease. The symptoms of JE include seizures, paralysis, coma, and mental status changes; residual neurological damage occurs in 50% of those with clinical disease. The case fatality rate is 30% in those with severe disease. Most symptomatic cases occur in children younger than 10 years and in the elderly. The areas at risk include most of Asia, Eastern Russia, some areas of the Western Pacific, and the Torres Strait Islands of Australia. The peak season is between April and October, during and just after the rainy season. The JE vaccine reduces the risk for disease among those who will be in a high-risk setting, as determined by the destination, duration, and season of travel. The JE vaccine licensed and available for use in the United States is Ixiaro, an inactivated Vero cell culture-derived vaccine. It was approved in May 2013 for use in children aged 2 months through 16 years, in addition to travelers older than 16 years of age. Other inactivated and live attenuated JE vaccines are manufactured and used in other countries, but are not licensed for use in the United States.
Rabies Vaccine
Rabies is found worldwide and contracted through the bite or saliva-contaminated scratch of infected animals. In parts of Africa, Asia, and Central and South America, canine rabies is highly endemic (Rabnet—www.who.int/rabies/rabnet/en/—provides country-specific animal and human data), where 40% of rabies occurs in children younger than 14 years. This increased risk is because children are attracted to animals, are more likely to be bitten, and may not report minor encounters with animals. Most cases of rabies in travelers occur through the bite of an infected dog, cat, or monkey (particularly those that live near temples in parts of Asia). Bats, mongooses, and foxes are other animals that can transmit disease.
The rabies vaccine is recommended for travelers to areas in which rabies is endemic and for those who will have occupational or recreational exposure (such as cavers), especially if access to medical care will be limited when traveling. The risk of a bite from a potentially rabid animal is up to 2% for travelers to the developing world. There are three types of inactivated virus vaccine available, which are administered prior to exposure in three doses at days 0, 7, and 21 or 28. Vaccination prior to exposure may not be completely protective; further doses are required if a high-risk bite occurs. The minimum age of administration is 1 year, and duration of protection is 2 years. Malaria chemoprophylaxis with mefloquine or chloroquine should begin 1 month after completing the rabies vaccine series to avoid interference with the immune response.
The manufacturers are currently unable to supply the rabies vaccine for preexposure prophylaxis and it is currently only available from distributors with existing stocks. There is no limitation in the supply of rabies immunoglobulin or vaccine for postexposure prophylaxis.
It is important to counsel travelers about animal avoidance, thorough cleansing of a bite wound with irrigation for at least 5 minutes, and the need for previously vaccinated individuals to seek additional vaccination on days 0 and 3 if an exposure occurs.
In the event of a bite in a nonvaccinated individual, rabies immunoglobulin and four doses of vaccine at days 0, 3, 7, and 14 are required, ideally within 24–48 hours after contact.
Yellow Fever Vaccine
Yellow fever is a flavivirus transmitted by mosquitoes, found in urban and rural areas in sub-Saharan Africa and equatorial South America. Of those infected with the virus, 15% have moderate to severe infection. The licensed 17D strain live attenuated vaccine is highly effective. It must be administered 10 days before travel to an endemic region to allow for the development of protective antibodies. It is required by many countries for reentry after travel to an endemic area, and receipt of the vaccine should be documented in the International Certificate of Vaccination that became available in December 2007 (wwwnc.cdc.gov/travel/yellowbook provides an updated list of countries in which yellow fever vaccination is recommended). For this reason, it is only administered at certified clinics. The vaccine is given subcutaneously, and a booster is required every 10 years (but immunity may be lifelong after a single dose). The recommended minimum age of administration is 9 months, and vaccine should not be administered to at-risk infants younger than 6 months, because of the increased risk of encephalitis (0.5–4 per 1000 vaccinees). The risk of severe vaccine-related disease is also higher in adult caretakers older than 60 years. The decision to immunize infants who are 6–8 months of age must balance the infant’s risk for exposure with the risk for vaccine-associated encephalitis. The vaccine should not be administered to individuals with egg allergy or immunosuppression (including a history of thymus disorder or thymectomy). A letter of medical exemption may be required for these travelers. In addition to age limitations, precautions to vaccination include asymptomatic HIV infection and CD4 T-lymphocyte count of 200–499 cells/mm3, pregnancy, and breast-feeding. Adverse effects include encephalitis (15 per million doses for those aged > 60 years) and multisystem disease (5 per million doses in older people).
Cholera Vaccine
The cholera vaccine is no longer produced in the United States and is no longer recommended for international travel, as the disease is rare in travelers. There are live and killed vaccines available in other countries, but these confer only slight protection.
Typhoid Vaccine
The risk of typhoid fever in travelers is 1–10:100,000, depending on the destination. Areas at risk include South Asia, West and North Africa, South America, and Latin America. Travelers to the Indian subcontinent are at greatest risk.
The vaccine is recommended for long-term travelers traveling to an endemic area, those traveling off standard tourist routes, immunocompromised travelers, those of south Indian ancestry, and patients with cholelithiasis. There are two vaccines available: a capsular polysaccharide (ViCPS) and a live attenuated (Ty21a) vaccine. The ViCPS is given intramuscularly 2 weeks prior to travel. The minimum age of administration for this vaccine is 2 years; efficacy is 75% over 2 years. The Ty21a is an oral vaccine given as four doses every second day, which needs to be completed more than 1 week prior to travel to be effective. It is licensed for children older than 6 years; efficacy is 80% over 5 years. It is contraindicated in immunodeficient populations. Antibiotics interfere with growth of the vaccine strain bacteria. Mefloquine, chloroquine, and prophylactic doses of atovaquone-proguanil can be given concurrently with the typhoid vaccine. Fever, headache, and severe local pain and swelling are reported with the ViCPS more frequently than with other vaccines. A recent recall of some lots of the ViCPS vaccine (because of low antigen content) may lead to some vaccine shortages.
Tuberculosis
Tuberculosis risk is increased for travelers, especially when visiting Africa, Asia, Latin America, and the former Soviet Union. The risk is higher in long-term travelers to countries with a high incidence of tuberculosis and is highest among health care workers. Bacillus Calmette-Guérin (BCG) vaccination is given soon after birth in many countries, but not in the United States. It protects against miliary and meningeal tuberculosis, but not against pulmonary disease, with efficacy only established in children younger than 1 year. It may be considered in children younger than 5 years who will be in a high-risk area for a prolonged period and have a negative tuberculin skin test (TST). It should not be given to immunosuppressed individuals. BCG is not widely available in the United States and may be administered in the destination country. A preferred alternative is that travelers to high-prevalence areas have a TST prior to travel and 3 months after return. It should be noted that live virus vaccines can create an anergic state, in which TSTs can be falsely negative. Therefore, TST should be performed on the same day as any live vaccine administration, or 28 or more days later.
The World Health Organization (WHO) has published recommendations related to tuberculosis and guidelines for tuberculosis prevention and management during air travel (www.who.int/docstore/gtb/publications/aircraft/PDF/98_256.pdf).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


