DIAGNOSTIC TESTING
1. Electroencephalography
Electroencephalography (EEG) is a noninvasive method for recording cerebral activity. The background patterns of the EEG vary by both age and clinical state of the subject, for example, infant, toddler, adolescent; awake, drowsy, or asleep. Intermittent activity often reflects disordered central nervous system (CNS) function. The EEG has its greatest clinical applicability in the evaluation of seizure disorders. An EEG may demonstrate “epileptiform activity,” that is, patterns that indicate risk for seizures and epilepsy, though not necessarily diagnostic of such. At times, however, the findings on an EEG are diagnostic, as in the hypsarrhythmic pattern of infantile spasms (West syndrome) or generalized three-cycle-per-second spike-wave pattern of absence epilepsy. Synchronized video recording with EEG has increased the utility of the test in assessing episodic disorders. EEG can be very useful in the evaluation of altered mental status and in some encephalopathies.
The EEG itself, in isolation, is rarely diagnostic but is one part of the child’s clinical picture. Routine EEG, obtained in the outpatient setting, is usually brief (< 30 minutes). Therefore, events of interest are usually not recorded. If the child is unable to cooperate, it may be impossible to obtain a study or the study may be uninterpretable due to artifact from movement, crying, etc. Medications used for sedation of an uncooperative child, especially barbiturates and benzodiazepines, may produce artifact in the tracing, which can confuse interpretation and may decrease the likelihood of recording abnormalities such as epileptiform discharges. Children without a history of epilepsy may have an abnormal EEG. EEG findings such as those occasionally seen in migraine, learning disabilities, or behavior disorders are often nonspecific and do not reflect structural brain damage. When questions arise regarding the clinical significance of EEG findings, consultation with a pediatric neurologist is appropriate.
Due to more prolonged recording duration, ambulatory EEG obtained over 24–48 hours can be useful in assessing events to ascertain if they are due to epileptic seizures. Likewise, recording the EEG during nocturnal polysomnographic studies can help differentiate between nonepileptic sleep-related events from nocturnal epileptic seizures arising from sleep.
Prolonged bedside EEG recordings are useful in the assessment of patients with altered mental status, suspected nonconvulsive status epilepticus, and drug-induced coma (for the treatment of increased intracranial pressure or status epilepticus), as well as infants with hypoxic ischemic encephalopathy. The EEG is less commonly used for determining so-called brain death (electrocerebral inactivity).
Continuous video-EEG monitoring, obtained as an inpatient, allows assessment of the patient with medically intractable epilepsy. The children are admitted to a specialized unit (epilepsy monitoring unit [EMU]) for up to a week or more. When children are admitted to the hospital, medications are often reduced or discontinued, increasing the likelihood of recording an event. Localization of the seizure focus by recording during seizures can lead to resective surgery for the patient who has failed medical therapy. Correlating video with EEG has also proven useful in characterizing spells that may or may not be seizures.
2. Evoked Potentials
Visual, auditory, or somatosensory evoked potentials (evoked responses) can be obtained by repetitive stimulation of the retina by light flashes, the cochlea by sounds, or a nerve by galvanic stimuli, which results in cortical response when recorded from the scalp surface using averaging techniques. The presence or absence of evoked potential waves and their latencies from the time of the stimuli are determined and can be useful in some specific situations although they are not routinely obtained for evaluation of neurologic disorders.
However, auditory evoked responses are now the standard for screening hearing in the neonate. Intraoperative somatosensory evoked potentials are often used during spine surgery to assist the surgeon during placement of instrumentation for identification of potentially reversible spinal cord injury. Similar techniques are used in other surgeries when there is risk of nerve injury such as craniofacial surgeries.
3. Lumbar Puncture
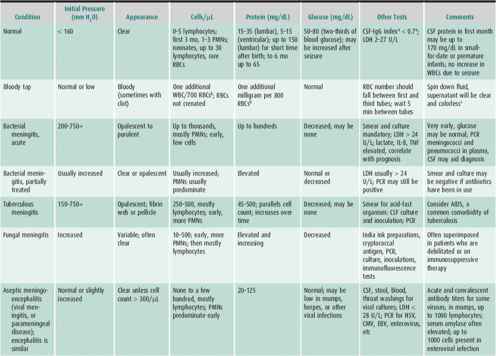
Assessing cerebral spinal fluid is a necessity in some clinical situations. Spinal fluid is usually obtained by inserting a small-gauge needle (eg, No. 22) through the L3–L4 intervertebral space into the thecal sac while the patient is lying in a lateral recumbent position. Radiographic guidance and sedation may be necessary in some patients. After an opening pressure is measured, fluid is removed to examine for evidence of infection, inflammation, or evidence of metabolic disorders (Table 25–2). Fluid is often sent for red and white cell counts, for determination of the concentrations of protein and glucose, for viral polymerase chain reaction (PCR), and for viral and bacterial cultures. In some cases, additional information is obtained with special staining techniques for mycobacteria and fungus and by testing for specific viral agents, antibody titer determinations, cytopathologic study, lactate and pyruvate concentrations, and amino acid and neurotransmitter analysis. Lumbar puncture is imperative when bacterial meningitis is suspected. Caution must be exercised, however, when signs of increased intracranial pressure (eg, papilledema) or focal neurologic signs are present that might indicate a substantial risk of precipitating tentorial or tonsillar herniation.
Table 25–2. Characteristics of cerebrospinal fluid in the normal child and in central nervous system infections and inflammatory conditions.
4. Genetic/Metabolic Testing
The diagnostic yield of genetic and metabolic evaluation of children with global developmental delay or intellectual disability (GDD/ID) depends on the specific testing done. Microarray testing is diagnostic in almost 8% of children with GDD/ID and in appropriate clinical situations; tests for metabolic disorders have a yield of up to 5%. Thus, focused assessments for genetic disorders should be part of the evaluation of the child with GDD/ID.
5. Electromyography & Nerve Conduction Velocity Testing
Electromyography (EMG) and nerve conduction velocity testing (NCV) are used for assessment of neuromuscular disorders such as spinal muscular atrophy, the Guillain-Barre syndrome, defects in neuromuscular transmission such as myasthenia gravis and infantile botulism, myopathies, acquired and hereditary neuropathies, and leukodystrophies, disorders associated with central as well as peripheral demyelination.
NCV is performed by introducing a small current into peripheral nerves using small discs overlying the nerves. The conduction velocity of the stimulus is calculated. EMG records spontaneous and volitional electrical activity of skeletal muscle tissue. It requires placement of tiny needles into selected muscles. While uncomfortable, the tests are not painful and sedation is only rarely necessary. For more details, refer to section “Disorders of Childhood Affecting Muscles” in the chapter.
PEDIATRIC NEURORADIOLOGIC PROCEDURES
1. Computed Tomography
CT scanning is a noninvasive technique, which allows visualization of intracranial contents by obtaining a series of cross-sectional (axial) roentgenograms. Serial images are obtained, which allow computation of x-ray absorption and computation of images which appear as serial slices. Current scanning techniques allow rapid acquisition of data, often without sedation. CT scanning is of high sensitivity (88%–96% of lesions larger than 1–2 cm can be seen) but low specificity (tumor, infection, or infarct may look the same). It is particularly useful for assessment of head trauma, allowing excellent visualization of intracranial blood. It allows visualization of the ventricular system to assess hydrocephalus. It is useful in determining presence of intracranial calcifications such as those associated with intrauterine infections, with tubers in patients with tuberous sclerosis complex, etc. Intravenous injection of iodized contrast media may be helpful in some situations but is not routinely used. CT angiography (CTA) is possible using contrast and specialized techniques to visualize vascular anatomy and can replace catheter angiography in evaluation of stroke. Radiation exposure is approximately the same as that from a skull radiographic series and must be considered when obtaining a CT scan.
2. Magnetic Resonance Imaging
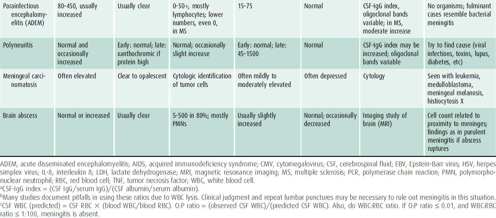
MRI is a noninvasive technique that provides high-resolution images of soft tissues. MRI uses the magnetic properties of certain nuclei to produce diagnostically useful signals (Table 25–3). The technique is based on detecting the response (resonance) of hydrogen proton to electromagnetic radiation. The strength of MRI signals varies with the relationship of water to protein and lipid in tissue. MRI can provide information about the histological, physiologic, and biochemical status of tissues as well as gross anatomic features. Sedation is often necessary for MRI scans in children who are unable to lie still for 45 minutes to avoid any movement artifact.
Table 25–3. Utility of MRI protocols.
MRI is used to assess a wide variety of neurologic disorders such as tumors, edema, ischemic and hemorrhagic lesions, vascular disorders, inflammation, demyelination, CNS infection, metabolic disorders, and degenerative processes. Because bone does not produce artifact in the images, the posterior fossa contents can be studied far better with MRI than with CT scans allowing brainstem, blood vessels, and the cranial nerves imaging.
Magnetic resonance angiography (MRA) or venography (MRV) is used to visualize large extra- and intracranial blood vessels (arterial and venous) without injection of dye, though they are not as sensitive as conventional angiography. The lack of radiation exposure is an advantage over CTA (see previous section). Perfusion-weighted imaging and diffusion-weighted imaging (DWI) (measuring random motion of water molecules) are used to evaluate brain ischemic penumbra and cytotoxic edema in acute stroke as well as toxic and metabolic brain disorders.
MR spectroscopy (MRS) assesses biochemical changes in CNS tissue, measuring signals of increased cellular activity and oxidative metabolism. For example, MRS can be used to identify brain tumors.
Newer applications of MRI allow for functional assessment of the CNS. Functional MRI (fMRI) is used to localize various brain functions such as language and motor by assessing blood oxygenation changes in an area of interest during language or motor tasks. The axonal tracts of neurologic pathways such as the optic radiations, or motor system, can be identified using diffusion tensor imaging (DTI). These techniques generally require a team involving a neuropsychologist and radiologist to derive specific paradigms for testing and evaluation of imaging, as well as a cooperative patient.
3. Positron Emission Tomography
Positron emission tomography (PET) is a nuclear medicine imaging technique that utilizes radiolabeled substrates such as intravenously administered fluorodeoxyglucose to measure the metabolic rate at given sites within the brain, producing three-dimensional reconstructions for localization of CNS function. These scans may be coregistered with a traditional CT scan or MRI allowing more precise localization of functional processes. It is proving to be very useful in preoperative evaluation for epilepsy surgery. PET is most often performed in the interictal state. The information from PET scan complements EEG, single-photon emission computerized tomography (SPECT), and MRI findings to aid in defining the epileptogenic zone (“focus”). PET coregistered with CT scans or MRI scans is used for assessing systemic tumors and is becoming increasingly available for use in evaluation of the CNS.
4. Single-Photon Emission Computerized Tomography
An application of nuclear medicine imaging, SPECT scans image cerebral blood flow using a radioactive tracer (typically technetium-99m) to produce multiple cuts similar to those obtained in CT scans. This allows virtual three-dimensional visualization of vascular blood flow. It is useful in assessment of patients for epilepsy surgery, aiding in identification of increased blood flow in a seizure focus during a seizure. In children with brain tumors, SPECT can help in differentiating tumor recurrence from post-treatment changes, in assessing the response to treatment, in directing biopsy, and in planning therapy. Regional cerebral blood flow can be assessed in children with strokes due to vascular stenosis and moyamoya disease.
5. Ultrasonography
Ultrasonography (US) offers a pictorial display of the varying densities of tissues in a given anatomic region by recording the echoes of ultrasonic waves reflected from it. US allows assessment of brain structures quickly with easily portable equipment, without ionizing radiation, and at about one-fourth the cost of CT scanning. Sedation is usually not necessary, and the procedure can be repeated as often as needed without risk to the patient. Ultrasonography has been used for in-utero diagnosis of hydrocephalus and other anomalies. In neonates, the thin skull and the open anterior fontanel have facilitated imaging of the brain, and ultrasonography is used to screen and follow infants at risk for intracranial hemorrhage. Other uses in neonates include detection of hydrocephalus, periventricular ischemic lesions, major brain and spine malformations, and calcifications. US of the neonatal spine can be used to determine the presence of anomalies at the lumbosacral level. Once the fontanels start to close, this modality is no longer useful due to inability to penetrate bone.
6. Cerebral Angiography
Arteriography remains useful in the diagnosis of cerebrovascular disorders, particularly cerebrovascular accidents and potentially operable vascular malformations. In some brain tumors, arteriography may be used to define the nature of tumors and for surgical planning. Since cerebral angiography uses traditional x-ray to produce images, there is significant exposure to ionizing radiation.
7. Myelography
Radiographic examination of the spine may be indicated in cases of spinal cord tumors, myelitis, or various forms of spinal dysraphism and in the rare instance of herniated disks in children. MRI has largely replaced sonography, CT, and myelography for examination of the spinal cord.
Beslow et al: Hemorrhagic transformation of childhood arterial ischemic stroke. Stroke 2011;42:941–946 [PMID: 21350202].
Cakir B et al: Inborn errors of metabolisms presenting in childhood. J of Neuroimaging 2011;21(2)e117–e133 [PMID: 21435076].
Dauud E et al: How MRI can contribute to the diagnosis of acute demyelinating encephalopathies in children. Neurosciences 2011;16(2):137–45 [PMID: 21427663].
Dahmoush HM, Vossough A, Roberts TP: Pediatric high-field magnetic resonance imaging. Neuroimaging Clin N Am. 2012;22:297–313 [PMID: 22548934].
Freilich ER, Gaillard WD: Utility of functional MRI in pediatric neurology. Curr Neurol Neurosci Rep 2010;10 (1):40–46 [PMID: 20425225].
Michelson DJ, Shevell MI, Sherr EH, Moeschler JB, Gropman AL, Ashwal S: Evidence report: genetic and metabolic testing on children with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2011 Oct 25;77(17):1629-1635 [PMID: 21956720].
Nigrovic LE, Malley R, Kuppermann N: Meta-analysis of bacterial meningitis score validation studies. Arch Dis Child 2012;97:799–805 [PMID: 22764093].
Pitt MC: Nerve conduction studies and needle EMG in very small children. Eur J Paediatr Neurol 2012;16:285–291 [PMID: 21840229].
Thakur NH, Lowe LH: Borderline low conus of medullaris of infant lumbar sonography: what is the clinical outcome and the role of neuroimaging follow-up? Pediatric Radiology 2011;41(4):483–487 [PMID: 21079942].
Townsend BA et al: Has pediatric CT at children’s hospitals reached its peak? AJ Roentgenology 2010;194(5):1194–1196 [PMID: 20410402].
Towsley K et al: Population based study of neuroimaging findings in children with cerebral palsy. Eur J Paediatric Neurol 2011;15(1):29–35 [PMID: 20869285].
Yock-Corrales A, Barnett P: The role of imaging studies for evaluation of stroke in children. Pediatr Emerg Care 2011;27:966–974 [PMID: 21975501].
DISORDERS AFFECTING THE NERVOUS SYSTEM IN INFANTS & CHILDREN
ALTERED STATES OF CONSCIOUSNESS
 General Considerations
General Considerations
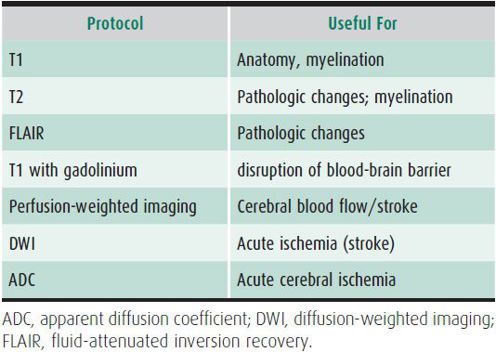
Many terms are used to describe the continuum from fully alert and aware, to complete unresponsiveness, including obtundation, lethargy, somnolence, stupor, light coma, and deep coma. Several scales have been used to grade the depth of unconsciousness (Table 25–4). The commonly used Glasgow Coma Scale is summarized in Table 12–5. Physicians should use one of these scales and provide further descriptions in case narratives (such as, “opens eyes with painful stimulus, but does not respond to voice”). These descriptions help subsequent observers quantify unconsciousness and evaluate changes in the patient’s condition.
Table 25–4. Gradation of coma.
The neurologic substrate for consciousness is the ascending reticular activating system in the brainstem, which extends the thalamus and paraventricular nucleus of the hypothalamus. Large lesions of the cortex, especially bilateral lesions, can also cause coma.
• Persistent vegetative state denotes a chronic condition in which there is preservation of the sleep-wake cycle but no awareness of self or the environment and no recovery of mental function. Sleep-wake cycles are present.
• Minimally conscious state denotes patients that do not meet criteria for persistent vegetative state. These patients occasionally may have purposeful movements.
• Brain death refers to patients in coma without brainstem reflexes or spontaneous respirations.
Conditions mistaken for coma:
• Locked-in syndrome describes patients who are conscious but have no access to motor or verbal expression because of massive loss of motor function of the pontine portion of the brainstem. Vertical eye movements may be preserved.
• Akinetic-mutism: Patient is aware, but does not initiate movement or follow commands. Caused by lesions of the frontal lobes.
• Catatonia refers to patients with psychiatric illness. Patients retain ability to maintain trunk and limb postures.
 Treatment
Treatment
A. Emergency Measures
The clinician must first stabilize the child using the ABCs of resuscitation. The airway must be kept open with positioning; endotracheal intubation is often considered. Breathing and adequate air exchange can be assessed by auscultation. Hand bag respiratory assistance with oxygen may be needed. Circulation must be ensured by assessing pulse and blood pressure. An intravenous line is always necessary. Fluids, plasma, blood, or even a dopamine drip (1–20 mcg/kg/min) may be required in cases of hypotension. Initial intravenous fluids should contain glucose until further assessment disproves hypoglycemia as a cause. An extremely hypothermic or febrile child may require vigorous warming or cooling to save life. The assessment of vital signs may signal the diagnosis. Slow, insufficient respirations suggest poisoning by hypnotic drugs; apnea may indicate diphenoxylate hydrochloride poisoning. Rapid, deep respirations suggest acidosis, possibly metabolic, as with diabetic coma; toxin exposure, such as that due to aspirin; or neurogenic causes, as in Reye syndrome. Hyperthermia may indicate infection or heat stroke; hypothermia may indicate cold exposure, ethanol poisoning, or hypoglycemia (especially in infancy).
The signs of impending brain herniation are another priority of the initial assessment. Bradycardia, high blood pressure, and irregular breathing are signs of severely increased intracranial pressure. Third nerve palsy (with the eye deviated down and out, and a “blown” pupil [unilateral pupillary dilation]) is a sign of impending temporal lobe or brainstem herniation. These signs suggest a need for hyperventilation to reduce cerebral edema, consideration of mannitol, prompt neurosurgical consultation, and head CT. If brainstem herniation or increased pressure is possible, intracranial monitoring may be necessary. Initial treatment of impending herniation includes keeping the patient’s head up (15–30 degrees) and providing moderate hyperventilation. The use of mannitol, diuretics, barbiturates, hypothermia, and drainage of cerebrospinal fluid (CSF) are more heroic measures covered in detail in Chapter 14.
A history obtained from parents, witnesses, or ambulance personnel is desirable. An important point is whether the child is known to have a chronic illness, such as diabetes, hemophilia, epilepsy, or cystic fibrosis. Recent acute illness raises the possibility of coma caused by viral or bacterial meningitis. Trauma is a common cause of coma. Lack of a history of trauma, especially in infants, does not rule it out. Abusive head trauma or an unwitnessed fall may have occurred. In coma of unknown cause, poisoning is always a possibility, especially in toddlers. Absence of a history of ingestion of a toxic substance or of medication in the home does not rule out poisoning as a cause.
Often the history is obtained concurrently with a brief pediatric and neurologic screening examination. After the assessment of vital signs, the general examination proceeds with a trauma assessment. Palpation of the head and fontanel, inspection of the ears for infection or hemorrhage, and a careful examination for neck stiffness are indicated. If circumstances suggest head or neck trauma, the head and neck must be immobilized so that any fracture or dislocation will not be aggravated. The skin must be inspected for petechiae or purpura that might suggest bacteremia, infection, bleeding disorder, or traumatic bruising. Examination of the chest, abdomen, and limbs is important to exclude enclosed hemorrhage or traumatic fractures.
Neurologic examination quantifies the stimulus response and depth of coma, such as responsiveness to verbal or painful stimuli. Are the eye movements spontaneous, or is it necessary to do the doll’s eye maneuver (rotating the head rapidly to see whether the eyes follow in a patient without neck trauma)? Motor and sensory examinations assess reflex asymmetries, Babinski sign, and evidence of spontaneous posturing or posturing induced by noxious stimuli (eg, decorticate or decerebrate posturing).
If the cause of the coma is not obvious, emergency laboratory tests must be obtained. Table 25–5 lists some of the causes of coma in children. Most comas (90%) in children have a medical (vs structural) cause. Infection is a common cause (30%). An immediate blood glucose, complete blood count, urine obtained by catheterization if necessary, pH and electrolytes (including bicarbonate), serum urea nitrogen, and aspartate aminotransferase and ammonia are initial screens. Urine, blood, and even gastric contents must be saved for toxin screen if the underlying cause is not obvious. Blood culture and lumbar puncture often are necessary to rule out CNS infection. However, papilledema is a relative contraindication to lumbar puncture. Often, a blood culture is obtained, antibiotics started, and imaging study of the brain done prior to a diagnostic lumbar puncture. If meningitis is suspected and a lumbar puncture is delayed or believed to be hazardous, antibiotics should be started and the diagnostic lumbar puncture done later. Tests that are helpful in obscure cases of coma include oxygen and carbon dioxide partial pressures, serum and urine osmolality, porphyrins, lead levels, general toxicology screen, serum amino acids, and urine organic acids. Hashimoto encephalopathy is a controversial, yet potentially treatable consideration. A formal metabolic consultation is also useful in this setting.
Table 25–5. Some causes of coma in childhood.
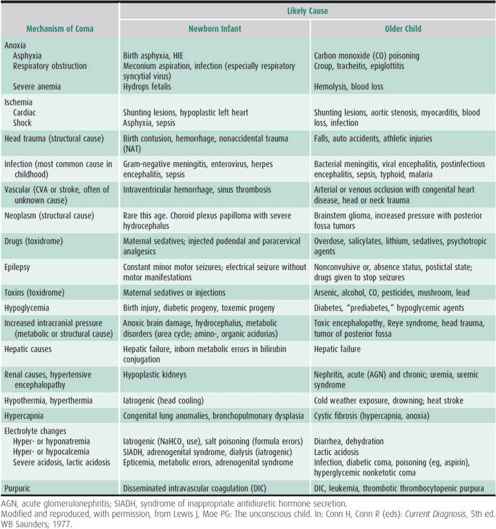
If head trauma or increased pressure is suspected, an emergency CT scan or MRI is necessary. CT is usually helpful as an initial screening examination, but MRI is more sensitive in finding anoxic brain injury early in the course. Bone windows on the former study or skull radiographs can be done at the same sitting. The absence of skull fracture does not rule out coma caused by closed head trauma. Injury that results from shaking a child is one example. Treatment of head injury associated with coma is discussed in detail in Chapter 12.
Rarely, an emergency EEG aids in diagnosing the cause of coma. Nonconvulsive status epilepticus or a focal finding as seen with herpes encephalitis (periodic lateralized epileptiform discharges) and focal slowing as seen with stroke or cerebritis are cases in which the EEG might be helpful. The EEG also may correlate with the stage of coma and add prognostic information. An EEG should be ordered if seizures are suspected. If obvious motor seizures have occurred, treatment for status epilepticus is given with intravenous drugs (see later section on Seizure Disorders).
B. General Measures
Vital signs must be monitored and maintained. The patient’s response to vocal or painful stimuli and orientation to time, place, and situation are monitored. Posture and movements of the limbs, either spontaneously or in response to pain, are serially noted. Pupillary size, equality, and reaction to light, and movement of the eyes to the doll’s eye maneuver or ice water caloric tests should be recorded (in patients without spine injury). Intravenous fluids can be tailored to the situation, as for treatment of acidosis, shock, or hypovolemia. Nasogastric suction is initially important. The bladder should be catheterized for monitoring urine output and for urinalysis.
 Prognosis
Prognosis
About 50% of children with nontraumatic causes of coma have a good outcome. In studies of adults assessed on admission or within the first days after the onset of coma, an analysis of multiple variables was most helpful in assessing prognosis. Abnormal neuro-ophthalmologic signs (eg, the absence of pupillary reaction or of eye movement in response to the doll’s eye maneuver or ice water caloric testing and the absence of corneal responses) were unfavorable. Delay in the return of motor responses, tone, or eye opening was also unfavorable. In children, the assessment done on admission is about as predictive as one done in the succeeding days. Approximately two-thirds of outcomes can be successfully predicted at an early stage on the basis of coma severity, extraocular movements, pupillary reactions, motor patterns, blood pressure, temperature, and seizure type. In patients with severe head trauma, a Glasgow Coma Scale ≤ 5, hypothermia, hyperglycemia, and coagulation disorders are factors associated with an increased risk of mortality. Other characteristics, such as the need for assisted respiration, the presence of increased intracranial pressure, and the duration of coma, are not significantly predictive. Published reports suggest that an anoxic (in contrast to traumatic, metabolic, or toxic) coma, such as that caused by near drowning, has a much poorer outlook.
Ashwal S et al: Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Dev Neurosci 2006;28:309 [PMID: 16943654].
Atabaki SM: Pediatric head injury. Pediatr Rev 2007;28:215 [PMID: 17545333].
Avner JR: Altered states of consciousness. Pediatr Rev 2006;27:331 [PMID: 16950938].
Castro-Gago M, Gómez-Lado C, Maneiro-Freire M, Eirís-Puñal J, Bravo-Mata M: Hashimoto encephalopathy in a preschool girl. Pediatr Neurol 2010 Feb;42(2):143–146 [PMID: 20117754].
Hosain SA et al: Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol 2005;32:162 [PMID: 15730895].
Odetola FO, Clark SJ, Lamarand KE, Davis MM, Garton HJ: Intracranial pressure monitoring in childhood meningitis with coma: a national survey of neurosurgeons in the United States. Pediatr Crit Care Med. 2011 Nov;12(6):e350-6. [PMID: 21263366].
Posner JB et al: Diagnosis of Stupor and Coma. Oxford University Press; 2007.
Shemie SD et al: Diagnosis of brain death in children. Lancet Neurol 2007;6:87 [PMID: 17166805].
Tude Melo JR: Mortality in children with severe head trauma: predictive factors and proposal for a new predictive scale. Neurosurgery 2010 Dec;67(6):1542–1547 [PMID: 21107185].
Worrall K: Use of the Glasgow Coma Scale in infants. Paediatr Nurs 2004;16:45 [PMID: 15160621].
SEIZURE DISORDERS (EPILEPSIES)
 General Considerations
General Considerations
A seizure is a sudden, transient disturbance of brain function, manifested by involuntary motor, sensory, autonomic, or psychic phenomena, alone or in any combination, often accompanied by alteration or loss of consciousness. Seizures can be caused by any factor that disturbs brain function. They may occur after a metabolic, traumatic, anoxic, or infectious insult to the brain (classified as symptomatic seizures), or spontaneously without prior known CNS insult. Genetic mutations are increasingly identified in many patients without prior known cause of seizures.
Repeated seizures without an evident acute symptomatic cause or provocation (eg, fever) are defined as epilepsy. The incidence is highest in the newborn period and higher in childhood than in later life, with another peak in the elderly. Prevalence flattens out after age 10–15 years. The chance of having a second seizure after an initial unprovoked episode in a child is about 50%. The risk of recurrence after a second unprovoked seizure is 85%. Sixty-five to seventy percent of children with epilepsy will achieve seizure remission with appropriate medication.
 Classification
Classification
The International League Against Epilepsy (ILAE) has established classifications of seizures and epilepsy syndromes. These were revised in 2010. Seizures are classified as either focal, previously called partial (with suspected seizure onset that can be localized to one part of the brain), or generalized (likely involving the whole brain or a network of the brain).
There are several types of generalized seizures that are recognized with the new classification: generalized tonic-clonic, absence (typical, atypical and with special features), myoclonic, myoclonic atonic, tonic, clonic, and atonic seizures. Focal seizures are no longer classified as simple and complex; this prior classification was based on loss of awareness which can be difficult to assess with some seizures, particularly if language areas are involved. With the new nomenclature, description of the seizure is most beneficial with suggested terms such as “without impairment of consciousness,” “with motor involvement,” or “hypomotor” seizure. This will allow better description and thus better classification of seizures.
Epilepsy syndromes are defined by the nature of the seizures typically present, age of onset, EEG findings, and other clinical factors. The prior terminology of idiopathic, symptomatic, and cryptogenic is no longer in use with the new ILAE classification system. The recommended terms are now “genetic,” to indicate a known or presumed genetic etiology. “Structural/metabolic” to indicate a known structural or metabolic etiology to an epilepsy syndrome; an example would be tuberous sclerosis or underlying stroke; and lastly “unknown” for those patients for whom a cause has not yet been identified.
1. Seizures & Epilepsy in Childhood
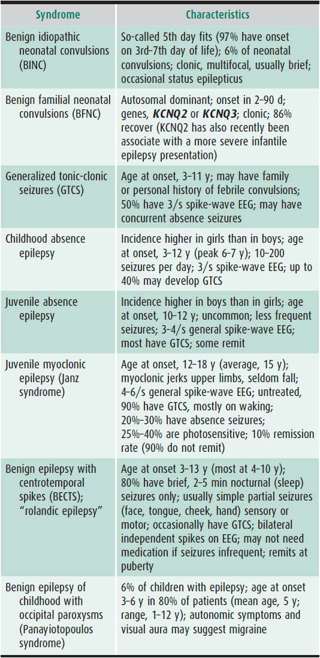
Characterizing the seizure is necessary for accurate diagnosis, which will determine the nature of further evaluation and treatment and help in prognostication (Tables 25–6 and 25–7).
Table 25–6. Seizures by age at onset, pattern, and preferred treatment.
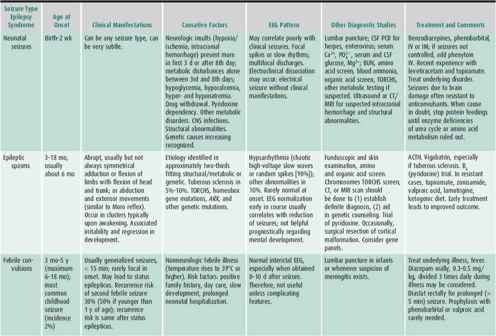
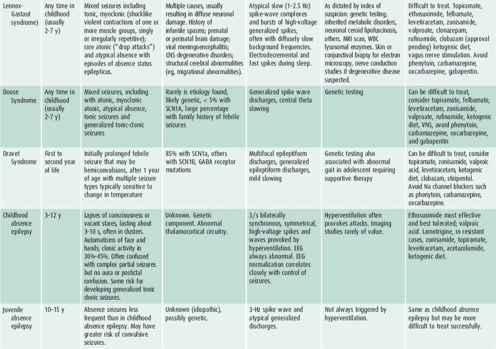
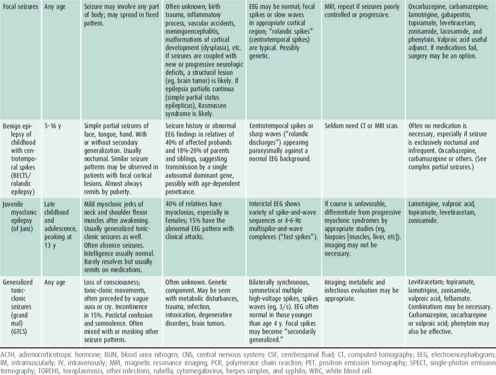
Table 25–7. Common childhood epileptic syndromes.
 Clinical Findings
Clinical Findings
A. History, Symptoms, and Signs
Seizures are stereotyped paroxysmal clinical events; the key to diagnosis is usually in the history. Not all paroxysmal events are epileptic. A detailed description of seizure onset is important in determining if an event is a seizure and if there is localized onset (partial or focal seizure). Events prior to, during, and after the seizure need to be described, although observers often recall little except generalized convulsive activity because of its dramatic appearance. An aura may precede the clinically apparent seizure and indicates focal onset. The patient may describe a feeling of fear, numbness or tingling in the fingers, or bright lights in one visual field. The specific symptoms may help define the location of seizure onset (eg, déjà vu suggests temporal lobe onset).
Often, the child does not recall or cannot define the aura, though the family may note alterations in behavior at the onset. Videotapes of events have been extremely useful.
Families may not immediately recall the details of the event but asking specific questions can help provide the details needed to determine the seizure type and, if partial, the site of onset. Did the patient become extremely pale before falling? Was the patient able to respond to queries during the episode? Was the patient unconscious or was there just impaired awareness? Did the patient fall stiffly or gradually slump to the floor? Was there an injury? How long did the tonic stiffening or clonic jerking last? Where in the body did the clonic activity take place? Which direction were head and eyes turned? Postictal states can be helpful in diagnosis. After complex partial and generalized convulsive seizures, postictal sleep typically occurs, but postictal changes are not seen after generalized absence seizures. Was there loss of speech after the seizure (suggesting left temporal lobe seizure) or was the patient able to respond and speak in short order? The parent may report lateralized motor activity (eg, the child’s eyes may deviate to one side or the child may experience dystonic posturing of a limb). Motor activity without impaired awareness supports the diagnosis of focal seizures as do impaired awareness and automatisms previously defined as a complex partial seizure.
In contrast, generalized seizures usually manifest with acute loss of consciousness, usually with generalized motor activity. Tonic posturing, tonic-clonic activity, or myoclonus (spasms) may occur. In children with generalized absence seizures, behavioral arrest may be associated with automatisms such as blinking, chewing, or hand movements, making it difficult to differentiate between absence seizures and partial seizures.
Description of the semiology of the event may help determine if the child experienced an epileptic seizure or a nonepileptic event mimicking or misinterpreted as an epileptic seizure. Frequently, the child presenting with a presumed first seizure has experienced unrecognized seizures before the event that brings the child to medical attention. In particular, partial and absence seizures may not be recognized except in retrospect. Thus, careful questioning regarding prior events is important in the child being evaluated for new onset of seizures.
B. Diagnostic Evaluation
The extent and urgency of the diagnostic evaluation is determined, in general, by the child’s age, the severity and type of seizure, whether the child is ill or injured, and the clinician’s suspicion about the underlying cause. Seizures in early infancy are often symptomatic. Therefore, the younger the child, the more extensive must be the diagnostic assessment.
It is generally accepted that every child with new onset of unprovoked seizures should be evaluated with an EEG and MRI, although this need not be done emergently. An EEG is very unlikely to yield clinically useful information in the child with a febrile seizure. Other diagnostic studies should be used selectively.
Metabolic abnormalities are seldom found in the well child with seizures. Unless there is a high clinical suspicion of serious medical conditions (eg, uremia, hyponatremia, hypocalcemia, etc), “routine” laboratory tests rarely yield clinically significant information. Special studies may be necessary in circumstances that suggest an acute systemic etiology for a seizure, for example, in the presence of apparent renal failure, sepsis, or substance abuse. Emergent imaging of the brain is usually not necessary in the absence evidence of trauma or of acute abnormalities on examination.
C. Electroencephalography
Appropriate use of EEG requires awareness of its limitations as well as its utility. The limitations of EEG even with epilepsy, for which it is most useful, are considerable. A routine EEG captures electrical activity during a very short period of time, usually 20–30 minutes. Thus, it is useful primarily for defining interictal activity (except for the fortuitous recording of a clinical seizure or in situations when seizures are easily provoked such as childhood absence epilepsy). A seizure is a clinical phenomenon; an EEG showing epileptiform activity may confirm and clarify the clinical diagnosis (for instance, defining an epilepsy syndrome), but it is only occasionally diagnostic.
1. Diagnostic value—The greatest value of the EEG in convulsive disorders is to help characterize seizure types and epilepsy syndromes. This can aid in prognostication and in selecting appropriate therapy (see Table 25–6). It is sometimes difficult to distinguish between hypomotor seizures due to generalized absence epilepsy vs localization-related epilepsy. The differing EEG patterns of these seizures will then prove most helpful. The presence of a mixed seizure EEG pattern in a child with clinically generalized convulsive seizures or only focal seizures may lead to identification of specific epilepsy syndromes and help the clinician select anticonvulsants effective for the seizure types identified by the EEG. Similarly, the EEG may help in diagnosing seizures in a young infant with minimal or atypical clinical manifestations; it may show hypsarrhythmia (high-amplitude spikes and slow waves with a chaotic background) in infantile spasms or the 1–4/s slow spike-wave pattern of the Lennox-Gastaut syndrome. The EEG may show focal slowing that, if constant, particularly when there are corresponding focal seizure manifestations and abnormal neurologic findings, will alert the physician to the presence of a structural lesion. In this case, brain imaging may establish the cause and help determine further investigation and treatment.
The EEG need not be abnormal in a child with epilepsy. Normal EEGs are seen following a first generalized seizure in one-third of children younger than age 4 years. The initial EEG is normal in about 20% of older children with epilepsy and in about 10% of adults with epilepsy. These percentages are reduced when serial tracings are obtained especially if sleep-deprived. Focal spikes and generalized spike-wave discharges are seen in 30% of close nonepileptic relatives of patients with epilepsy.
2. Prognostic value—EEG following febrile seizures is almost always normal and is not clearly predictive of subsequent seizures and therefore is not useful in these situations. Hypsarrhythmia or slow spike and wave patterns support the diagnosis of infantile spasms and Lennox-Gastaut syndrome, respectively. Both are expressions of diffuse brain dysfunction (epileptic encephalopathy) and are generally of grave significance. Central-temporal (rolandic spikes) and occipital spike-wave activity (occipital paroxysms) are the EEG correlates of idiopathic focal epilepsies of childhood.
Following successful treatment, an abnormal EEG may become normal and may aid in the decision to discontinue medications but is not always present. Normalization can also be seen in infants with infantile spasms who have been successfully treated and, less commonly, in children with other epileptic encephalopathies.
EEG should be repeated when the severity and frequency of seizures increase despite adequate anticonvulsant therapy, when the clinical seizure pattern changes significantly, or when progressive neurologic deficits develop. Emergence of new focal or diffuse slowing may indicate a progressive lesion or a neurodegenerative disorder.
The EEG may be helpful in determining when to discontinue anticonvulsant therapy. The presence or absence of epileptiform activity on the EEG prior to withdrawal of anticonvulsants after a seizure-free period of 2 years on medications has been shown to correlate with the degree of risk of seizure recurrence. However, persistent focal epileptiform discharges are common in children with so-called benign epilepsies until they resolve spontaneously in adolescence and may not be considered a reason to not reduce anticonvulsants.
 Differential Diagnosis
Differential Diagnosis
It is extremely important to be accurate in the diagnosis of epilepsy and not to make the diagnosis without ample proof. To the layperson, epilepsy often has connotations of brain damage and limitation of activity. A person so diagnosed may be excluded from certain occupations in later life. It is often very difficult to change an inaccurate diagnosis of many years’ standing.
Misinterpretation of behaviors in children is the most common reason for misdiagnosis. Psychogenic nonepileptic seizures are much less common in children than in adults but must be considered even in the young or cognitively impaired child. The most commonly misinterpreted behaviors are inattention in school-aged children with attention disorders, stereotypies in children with autistic spectrum disorder, sleep-related movements, habit movements such as head-banging and so-called infantile masturbation (sometimes referred to as gratification movements), and gastroesophageal reflux in very young (often impaired) infants. Some of the common nonepileptic events that mimic seizure disorder are listed in Table 25–8.
Table 25–8. Nonepileptic paroxysmal events.

 Complications & Sequelae
Complications & Sequelae
A. Psychosocial Impact
Emotional disturbances, especially depression but also anxiety, anger, and feelings of guilt and inadequacy, often occur in the patient as well as the parents of a child with epilepsy. Actual or perceived stigma as well as issues regarding “disclosure” are common. There is an increased risk of suicide in people with epilepsy. Schools often limit activities of children with epilepsy inappropriately and stigmatize children by these limitations.
Epilepsy with onset in childhood has an impact on adult function. Adults with early onset of epilepsy are less likely to complete high school, have less adequate employment, and are less likely to marry. This is also true of populations with well-controlled epilepsy. Persistent epilepsy results in significant dependence; even when epilepsy is successfully treated, patients with long-standing epilepsy often do not become independent due to driving restrictions and safety concerns.
B. Cognitive Delay
Untreated seizures can have an impact on cognition and memory. Clearly, epileptic encephalopathy (ie, regression in cognitive ability and development associated with uncontrolled seizures) does occur, particularly in young children with catastrophic epilepsies such as infantile spasms (West syndrome), Dravet syndrome, and Lennox-Gastaut syndrome. The impact of persistent partial seizures on development is less clear although persistent temporal lobe seizures in adults are associated with cognitive dysfunction. It is not likely that interictal epileptiform activity contributes to cognitive impairment in older children, although increased epileptiform burden has been demonstrated to cause mild cognitive problems in some disorders previously thought to be benign, such as benign epilepsy with central temporal spikes (BECTS). Continuous epileptiform activity in sleep is associated with Landau-Kleffner syndrome (acquired epileptic aphasia) and the syndrome of Electroencephalographic Status Epilepticus in Sleep (ESES) which are associated with cognitive decline.
Pseudodementia may occur in children with poorly controlled epilepsy because their seizures interfere with their learning. Depression is a common cause of impaired cognitive function in children with epilepsy. Anticonvulsants are less likely to cause such interference at usual therapeutic doses, although phenobarbital topiramate and zonisamide may produce cognitive impairment, reversible on discontinuing the medication. Psychosis can also occur after seizures or as a side effect of medications.
C. Injury and Death
Children with epilepsy are at far greater risk of injuries than the general pediatric population. Physical injuries, especially lacerations of the forehead and chin, are frequent in astatic or akinetic seizures (so-called drop attacks), necessitating protective headgear. In all other seizure disorders in childhood, injuries as a direct result of an attack are not as common although drowning, injuries related to working in kitchens, and falls from heights remain potential risks for all children with active epilepsy. It is therefore extremely important to stress “seizure precautions,” in particular, water safety. Bathrooms are a particularly dangerous room for people with uncontrolled epilepsy as the room is usually small and has many hard surfaces. Showers are recommended over bathing as they decrease the likelihood of drowning. Appropriate supervision is recommended.
The greatest fear of a parent of a child with new-onset of epilepsy is the possibility of death or brain injury. There is an increased risk of premature death in patients with symptomatic epilepsy, especially those who have not achieved seizure control. Most of the mortality in children with epilepsy is related to the underlying neurologic disorder, not the seizures. Sudden unexpected death with epilepsy (SUDEP) is a rare event in children. Although children with epilepsy have an increased risk of death, SUDEP occurs in only 1–2:10,000 patient-years. The greatest risk for SUDEP is in children with medically uncontrolled epilepsy, especially with symptomatic epilepsy (associated with identifiable CNS etiology). There is no current proven strategy to prevent SUDEP other than seizure control. The mechanism for SUDEP is unclear but is probably most commonly related to either cardiac arrhythmia induced by a seizure or sudden respiratory insufficiency. Vigorous attempts to control intractable seizure disorders remain the most important approach. Identifying life-threatening disorders (eg, identifying patients with cardiac arrhythmias, especially prolonged QT syndrome) as the cause of misdiagnosed epilepsy is clearly of utmost importance. While SUDEP is rare, increased mortality in children with epilepsy should be mentioned when counseling families.
 Treatment
Treatment
The ideal treatment of acute seizures is the correction of specific causes. However, even when a biochemical disorder, a tumor, meningitis, or another specific cause is being treated, anticonvulsant drugs are often still required.
A. First Aid
Caregivers should be instructed to protect the patient against self-injury. Turning the child to the side is useful for preventing aspiration. Thrusting a spoon handle, tongue depressor, or finger into the clenched mouth of a convulsing patient or trying to restrain tonic-clonic movements may cause worse injuries than a bitten tongue or bruised limb and could potentially become a choking hazard. Parents are often concerned that cyanosis will occur during generalized convulsive seizures but it is rare for clinically significant hypoxia to occur. Mouth-to-mouth resuscitation is rarely necessary and is unlikely to be effective.
For prolonged seizures (those lasting over 5 minutes), acute home treatment with benzodiazepines such as rectal diazepam gel (Diastat) or intranasal midazolam may be administered to prevent the development of status epilepticus and has proven to be safe even when administered by nonmedical professionals, including teachers and day care providers, when appropriately instructed.
B. Antiepileptic Drug (AED) Therapy
1. Drug selection—Treat with the drug appropriate to the clinical situation, as outlined in Table 25–9.
Table 25–9. Guide to AED use.
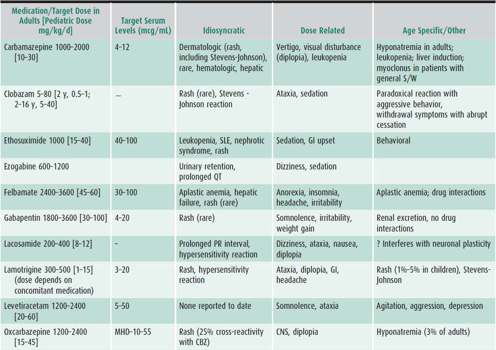
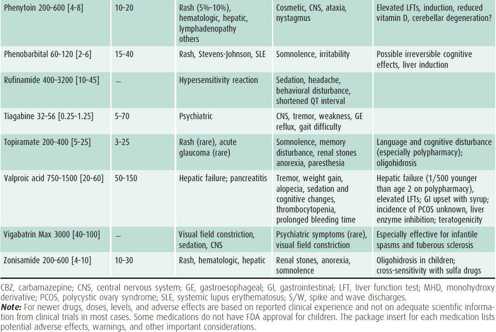
2. Treatment strategy—The child with a single seizure has a 50% chance of recurrence. Thus, it is usually not necessary to initiate AED therapy until the diagnosis of epilepsy is established, that is, there is a second seizure. The seizure type and epilepsy syndrome as well as potential side effects will determine which drug to initiate (see Table 25–9). Start with one drug in moderate dosage and increase the dosage until seizures are controlled. If seizures are not controlled on the maximal tolerated dosage of one major AED, gradually switch to another before using two-drug therapy. Polytherapy (ie, the use of more than two medications concurrently) is rarely sufficiently effective to warrant the considerable risk of adverse side effects from the synergistic impact of multiple medications.
Dosages and usual target serum levels of commonly prescribed AEDs are listed in Table 25–9. Individual variations must be expected, both in tolerance and efficacy. The therapeutic range may also vary somewhat with the method used to determine levels, and published levels are not always reflective of clinical efficacy and tolerability.
3. Long-term management and discontinuation of treatment—AEDs should be continued until the patient is free of seizures for at least 1–2 years. In about 75% of patients, seizures will not recur following discontinuation of medication after 2 years of remission. Variables such as younger age at onset, normal EEG, undetermined etiology, and ease of controlling seizures carry a favorable prognosis, whereas identified etiology, later onset, continued epileptiform EEG, difficulty in establishing initial control of the seizures, polytherapy, generalized tonic-clonic or myoclonic seizures, as well as an abnormal neurologic examination are associated with a higher risk of recurrence. Most AEDs (with the exception of barbiturates and clonazepam) can be withdrawn over 6–8 weeks. There does not appear to be an advantage to slower withdrawal.
Recurrent seizures affect up to 25% of children who attempt withdrawal from medications. Recurrence of seizures is most likely within 6–12 months of discontinuing medications. Therefore, seizure safety precautions will need to be reinstituted, including driving restriction. If seizures recur during or after withdrawal, AED therapy should be reinstituted and maintained for at least another 1–2 years. The vast majority of children will again achieve remission of their seizures.
C. Alternative Treatments
1. Adrenocorticotropic hormone (ACTH) and corticosteroids—ACTH is indicated for treatment of infantile spasms. The utility of other immunotherapy is less clear. Duration of ACTH therapy is guided by cessation of clinical seizures and normalization of the EEG. Oral corticosteroids and intravenous immune globulin (IVIG) are occasionally used for pharmacoresistant epilepsy. However, dosing regimens and indications are not well established.
Landau-Kleffner syndrome (acquired epileptic aphasia) is reported to respond to oral steroid treatment. Anecdotal reports of use of immunosuppression in other patients have been published but no controlled clinical trials have been performed.
Precautions: Give additional potassium, guard against infections, provide GI prophylaxis, follow for possible hypertension, and discuss the cushingoid appearance and its disappearance. Do not withdraw oral corticosteroids suddenly. Side effects in some series occur in up to 40% of patients, In some regions of the country, prophylaxis against Pneumocystis infection may be required. Careful and frequent follow-up is necessary. Visiting nurse services can be very helpful in surveillance such as monitoring blood pressure, weight, and potential adverse effects.
2. Ketogenic diet—Fasting has been described to stop seizures for centuries and a diet high in fat and low in protein and carbohydrates will result in ketosis and simulate a fasting state. Fatty acids replace glucose as a source of energy for cellular metabolism. Such a diet has been observed to decrease and even control seizures in some children. A ketogenic diet should be considered for children with pharmacoresistant epilepsy. This diet should be monitored very carefully to ensure sufficient protein for body maintenance and growth as well as appropriate vitamin and mineral supplementation. Recent reports suggest efficacy with a modified Atkins diet or a low-glycemic index diet in older and higher functioning children who will not accept the ketogenic diet. A prepared commercial formula is available for children receiving tube feedings.
The mechanism for the anticonvulsant action of the ketogenic diet is not understood. The ketogenic diet requires close adherence full cooperation of all family members is required. However, when seizure control is achieved by this method, acceptance of the diet is usually excellent. Access to other families and patients via Internet has provided support and is particularly useful for providing increased variation of meals for families.
As with all therapies, potential adverse effects can occur with the ketogenic diet. These include acidosis and hypoglycemia, particularly on initiation of the diet. Thus, it is prudent to admit the child for initiation of the diet after screening laboratory studies are performed to rule out underlying metabolic disorders. Renal stones, pancreatitis, and acidosis can occur. In addition, vitamin and minerals need to be followed carefully to avoid deficiencies especially carnitine, iron, and vitamin D.
3. Vagus nerve stimulator (VNS)—The VNS is a pacemaker-like device that is implanted below the clavicle on the left and attached to the left vagus nerve. A cycle of electrical stimulation of the nerve is established (typically 30 seconds of stimulation every 5 minutes), which has an antiepileptic effect, reducing seizures by at least 50% in over half the children so treated. In addition, an emergency mode that is activated by the use of a magnet may interrupt a seizure (ie, an anticonvulsant effect). For patients with sufficient warning of an impending seizure, the device can be activated with abortion of the seizure. Many patients also experience an improvement in learning and behavior with use of this device. With current technology, the battery in the stimulator will last 7 or more years in many patients.
D. Surgery
An evaluation for epilepsy surgery is indicated for all children with medically intractable partial epilepsy. The evaluation and surgery should be performed at a center with expertise in epilepsy surgery and which has a dedicated neurosurgeon, epileptologists, neuropsychologists, and physiatrists with experience in epilepsy surgery.
The first surgery for treatment of epilepsy took place over 100 years ago, and surgery is now established as an appropriate treatment option for adults and children with epilepsy refractory to medical treatment. Evaluation for possible surgical treatment should begin as soon as it is apparent that a child with focal onset seizures is not responding to standard therapy. Medication resistant (“refractory”) epilepsy is usually defined as failure of two or three anti-epileptic drugs alone or as combination therapy to control seizures. Advances in technology allow for definition and removal of the epileptogenic focus even in young infants. Many centers now have access to video-EEG monitoring, positron emission tomography (PET), single photon emission computerized tomography (SPECT), and similar noninvasive techniques that can be used to identify the “ictal onset zone” for seizures such as a focal cortical dysplasia that may amenable to resection. Freedom from seizures is reported in as many as 80% who have been treated surgically. Some children without an identifiable onset to seizure may qualify for other types of surgery such as corpus callosotomy that aim to reduce seizure burden.
E. General Management of the Child with Epilepsy
1. Education—The initial diagnosis of epilepsy is often devastating for families. The patient and parents must be helped to understand the nature of epilepsy and its management, including etiology, prognosis, safety issues, and treatment options.
Excellent educational materials are available for families of a child with epilepsy, both in print and online. Two excellent web sites are http://www.epilepsyfoundation.org and http://www.epilepsy.com. Materials on epilepsy—including pamphlets, monographs, films, and videotapes suitable for children and teenagers, parents, teachers, and medical professionals—may be purchased through the Epilepsy Foundation: 8301 Professional Place, Landover, MD 20785; (800) 332–1000. The foundation’s local chapter and other community organizations are able to provide guidance and other services. Support groups exist in many cities for older children and adolescents and for their parents and others concerned.
2. Privileges and precautions in daily life—“No seizures and no side effects” is a motto established by the Epilepsy Foundation. The child should be encouraged to live as normal a life as possible. Children should engage in physical activities appropriate to their age and social group. After seizure control is established, swimming is generally permissible with a buddy system or adequate lifeguard coverage. Scuba diving and high climbing without safety harness is generally not allowed. There are no absolute contraindications to any other sports, although some physicians recommend against contact sports. Physical training and sports are usually to be welcomed rather than restricted. There is some literature that suggests that exercise decreases overall seizure burden. Driving is discussed in the next section.
Emotional disturbances, especially depression, are not uncommon, particularly in adolescents with epilepsy, and need to be treated. Loss of sleep should be avoided as sleep deprivation can be a trigger for seizures. Alcohol intake should be avoided because it may precipitate seizures. Prompt attention should be given to intercurrent illnesses as these can trigger seizures.
Although every effort should be made to control seizures, treatment must not interfere with a child’s ability to function normally. A child may do better having an occasional mild seizure than being so heavily sedated that function at home, in school, or at play is impaired. Therapy and medication adjustment often require much art and fortitude on the physician’s part. Some patients with infrequent seizures, especially if only nocturnal partial seizures (eg, Rolandic seizures) may not need treatment with AEDs.
3. Driving—Driving becomes important to most young people at age 15 or 16 years. Restrictions for persons with epilepsy and other disturbances of consciousness vary from state to state. In most states, a learner’s permit or driver’s license will be issued to an individual with epilepsy if he or she has been under a physician’s care and free of seizures for at least 1 year provided that the treatment or basic neurologic problems do not interfere with the ability to drive. A guide to this and other legal matters pertaining to persons with epilepsy is published by the Epilepsy Foundation, and its legal department may be able to provide additional information.
4. Pregnancy—Contraception (especially interaction of oral contraceptive with some AEDs), childbearing, potential teratogenicity of AEDs, and the management of pregnancy should be discussed as soon as appropriate with the adolescent young woman with epilepsy. Daily use of vitamin preparations containing folic acid is recommended. For the pregnant teenager with epilepsy, management by an obstetrician conversant with the use of AEDs in pregnancy is appropriate. The patient should be cautioned against discontinuing her medications during pregnancy. The possibility of teratogenic effects of AEDs, such as facial clefts (two to three times increased risk), must be weighed against the risks from seizures. All AEDs appear to have some risk for teratogenicity, although valproate carries a particularly high risk for spinal dysraphism as well as being associated with cognitive issues in children exposed to valproate in utero. Dosing may need to be adjusted frequently during pregnancy as blood volume expands. Frequent AED blood levels may be helpful in making these adjustments.
5. School intervention and seizure response plans—Schools are required by federal law to work with parents to establish a seizure action plan for their child with epilepsy. A template for such a plan is available on the Epilepsy Foundation website at http://www.epilepsyfoundation.org/programs/upload/snactionplan.pdf. These plans usually require the approval of the child’s physician. Schools are sometimes hesitant to administer rectal valium or to activate the vagal nerve stimulator. Often, information from the physician, especially that obtained from the Epilepsy Foundation website, will relieve anxieties. School authorities should be encouraged to avoid needless restrictions and to address the emotional and educational needs of all children with disabilities, including epilepsy. The local affiliates of the Epilepsy Foundation can often provide support for families in their interactions with the school.
2. Status Epilepticus
Status epilepticus is usually defined as a clinical or electrical seizure lasting at least 15 minutes, or a series of seizures without complete recovery over a 30-minute period. After 30 minutes of seizure activity, hypoxia and acidosis occur, with depletion of energy stores, cerebral edema, and structural damage. Eventually, high fever, hypotension, respiratory depression, and even death may occur. Status epilepticus is a medical emergency. Aggressive treatment of prolonged seizures may prevent development status epilepticus. It is generally recommended that treatment with benzodiazepines at home for prolonged seizures be initiated, 5 minutes after onset of a seizure. There are currently several forms of benzodiazepines that can be administered safely at home, rectal valium, intranasal midazolam, sublingual lorazepam, and intramuscular diazepam.
Status epilepticus is classified as (1) convulsive (the common tonic-clonic, or grand mal, status epilepticus) or (2) nonconvulsive (characterized by altered mental status or behavior with subtle or absent motor components). Absence status, or spike-wave stupor, and focal status epilepticus are examples of the nonconvulsive type. An EEG may be necessary to aid in diagnosing nonconvulsive status because patients sometimes appear merely stuporous and lack typical convulsive movements.
 Treatment
Treatment
For treatment options, see Table 25–10.
Table 25–10. Status epilepticus treatment.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








