Transfusion Medicine
Cassandra D. Josephson
Transfusion of blood components is essential for treating children with various neonatal and pediatric disorders. Neonatal and pediatric transfusion practices can be classified into two age groups: infants up to 4 months of age and infants/children greater than 4 months of age. This chapter addresses specific aspects of pediatric transfusion medicine, namely the constituents of blood, indications, physician ordering, component preparation in blood banks, administration, and potential adverse events in each age group. It is important to note that prior to ordering or administering any blood product or component, informed consent explaining indications, treatment plan(s), benefits, and risks must be obtained unless a transfusion is required in an emergency.
PACKED RED BLOOD CELLS (PRBCS)
 COMPOSITION AND VOLUME
COMPOSITION AND VOLUME
Packed red blood cells (pRBCs) are the most commonly transfused component of whole blood. They are derived by centrifugation or, less frequently, are acquired directly from the donor by apheresis techniques, known as non-whole blood derived. See Table 442-1 for types of pRBC products, volume per unit, dosage, hematocrit and storage solution, and storage periods. Diverse anticoagulant preservative solutions are used to conserve red blood cells and may uniquely affect each neonatal/pediatric recipient. While additive solutions (AS) have evolved to extend the shelf life of red cells, the safety of the concentration of adenine and mannitol in AS and their associated renal toxicity when given to neonates has come into question. Additionally, the use and safety of AS-preserved pRBCs in massive transfusions for trauma, cardiac surgery, or exchange transfusions for infants less than 4 months old has not been established. The use of AS-preserved red cell units in this population in specific clinical situations must be approached with caution.2-5
 INDICATIONS FOR TRANSFUSION OF PRBCS IN PATIENTS LESS THAN 4 MONTHS OF AGE
INDICATIONS FOR TRANSFUSION OF PRBCS IN PATIENTS LESS THAN 4 MONTHS OF AGE
Packed RBCs are the most commonly transfused product during the neonatal period,8 chiefly for symptomatic anemia or after a 10% reduction of blood volume from iatrogenic or other losses. Guidelines for managing pRBC transfusion in neonates have been published9-13 and are primarily based on clinical practice rather than on evidence. See eTable 442.1  for the most recently published guidelines.14
for the most recently published guidelines.14
 INDICATIONS FOR TRANSFUSION OF PRBCS IN PATIENTS OLDER THAN 4 MONTHS OF AGE
INDICATIONS FOR TRANSFUSION OF PRBCS IN PATIENTS OLDER THAN 4 MONTHS OF AGE
The transfusion indications for pediatric patients older than 4 months are similar to adult pRBC transfusions. However, significant differences exist, namely blood volume, the ability to tolerate blood loss, and age-appropriate hemoglobin and hematocrit levels. A reduction of red cell mass, resulting from surgery, anemia of chronic diseases, hematologic malignancies, or slowly developing anemia is the most common indications for a red cell transfusion in this population. See eTable 442.2  (online text) for transfusion parameters for children older than 4 months of age.14
(online text) for transfusion parameters for children older than 4 months of age.14
 ORDERING
ORDERING
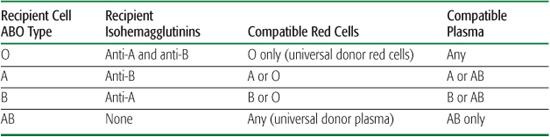
ABO and Rh compatibility tests as required by the AABB (previously known as the American Association of Blood Banks) are listed in Table 442-2.15 At the earliest indication for a nonurgent pRBC transfusion, a type and screen should be ordered to determine the recipient’s ABO/Rh antigen type. The recipient’s serum should be “screened” with the indirect antiglobulin test (IAT) for unexpected alloantibodies that may have resulted from previous receipt of red cells. A type and crossmatch is performed to determine the patient’s type of ABO and Rh antigens, to screen for antibodies to unexpected red blood cell minor antigens, and to identify compatible red cells for transfusion.16 Cross-matched red cells may be issued more easily and rapidly when the antibody screen is negative and the crossmatch is nonreactive. A positive antibody screen requires assessing the significance of the antibody detected (ie, its capability of causing intravascular or extravascular hemolysis) prior to selecting donor red cell units for crossmatch.
Once the specificity of the antibody is determined, antigen-negative red cells can be selected. Exclusive of emergent transfusions, crossmatch testing must be done prior to issuing any red cell unit. In the event of an urgent red cell transfusion, when there is insufficient time to obtain ABO/Rh typing, a physician may order the emergency release of this prod-uct(s). For an emergency release order, group O (universal donor) and Rh-negative, uncrossmatched red cells are issued.  In less emergent scenarios, screening and cross-matching are not performed prior to product dispensation, yet type-compatible blood (O type for A, B, or AB recipients; A type for A recipients; and B type donor cells for B recipients) is issued by the blood bank.
In less emergent scenarios, screening and cross-matching are not performed prior to product dispensation, yet type-compatible blood (O type for A, B, or AB recipients; A type for A recipients; and B type donor cells for B recipients) is issued by the blood bank.
 DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
The standard dose of red cells for infants less than 4 months of age is approximately 10 ml/kg, administered over a 2- to 4-hour period. This dose and rate can be transfused safely without additional processing, such as washing or resuspending the cells in another solution. Premature infants with severe hepatic or renal compromise require removal of the additive solution and resuspension of RBCs in saline or albumin. In larger volumes, such as for exchange transfusions, cardiac surgery, extracorporeal membranous oxygenation (ECMO), or massive transfusion, RBCs in extended storage media are not recommended.17 Infants older than 4 months of age and children with good cardiac and vascular function can tolerate an infusion of 10 to 20 ml/kg of pRBCs. As in younger infants, the pRBCs may be infused over 2 to 4 hours unless underlying disease deems other infusion strategies. The older child’s increased blood and plasma volume render the anticoagulant preservative solutions less important. A nonbleeding patient who receives a 10 ml/kg dose of pRBC will likely achieve a rise in hemoglobin concentration of 1 to 2 g/dl, or a 3% to 6% increase in hematocrit level. If the transfusion results in a poor increment, one can suspect peripheral destruction of the red cells or blood loss.
Table 442-1. Packed Red Blood Cell Products50
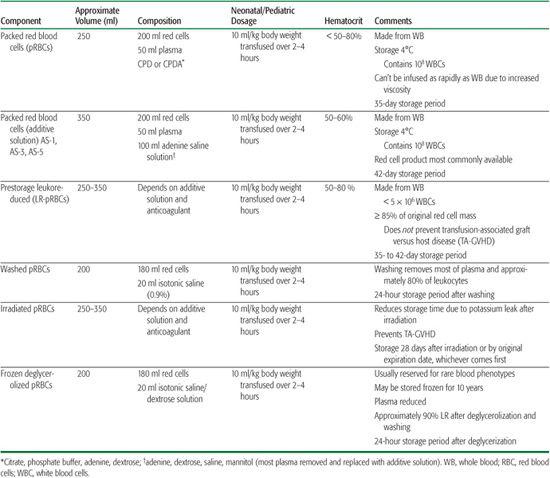
Table 442-2. ABO-Compatible Blood Products
 PREPARATION
PREPARATION
For the prevention of transfusion errors, accurate component and recipient identification is imperative; the blood sample must be labeled with the recipient’s name and hospital identification number as taken from, and thus exactly matching, the hospital identification band always worn by the patient. Initial patient testing on the plasma or serum from either the infant or mother must include ABO and D typing of their red blood cells (RBCs) and a screen for unexpected RBC antibodies. Nonetheless, prior to issuing nongroup type O RBCs, the infant’s plasma or serum is tested to detect passively acquired maternal anti-A or anti-B. In the presence of an antibody, ABO-compatible RBCs are administered until the acquired antibody is no longer detected.14
 ADMINISTRATION
ADMINISTRATION
At the bedside, one must verify the recipient’s identity, matching the identity and hospital identification number with the corresponding name and number on the donor unit. Such corroboration is vital for avoiding a mistransfusion attributed to clerical errors, which account for a majority of fatal hemolytic transfusion reactions.23 All blood components are administered through a standard in-line filter that removes particles that may have accumulated during storage. Prior to initiating a transfusion, the patient’s vital signs are measured; the infusion is then started slowly while observing the patient for a potential transfusion reaction over the first 15 minutes. If a transfusion reaction is suspected, the infusion is slowed further or halted altogether. A slow infusion rate (∼ 2 ml/kg per hour) is recommended for patients with severe anemia or heart failure, thereby reducing the risk of volume overload. Patients who experience acute hemorrhage or hypovolemia require rapid infusion of pRBCs to restore intravascular volume. Regardless of the indication, once a transfusion has been initiated, it must be completed within 4 hours to minimize the risk of bacterial contamination, which increases exponentially when pRBCs are subjected to room temperature for longer than 4 hours. RBCs should not be infused too rapidly or pushed through a very small needle, as this may cause hemolysis and a transfusion reaction. 
PLATELETS
 COMPONENT AND VOLUME
COMPONENT AND VOLUME
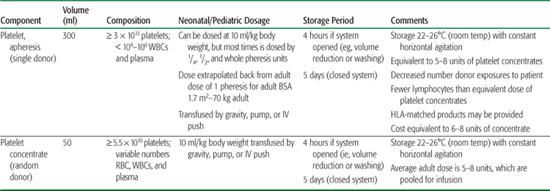
In the United States, both pooled platelet concentrates (known as random donor or whole blood–derived platelets) and apheresis platelets, known as single-donor platelets, are available. Platelet concentrates are derived from whole blood drawn from a donor, whereas single-donor platelets are collected via an apheresis machine that returns the remaining whole blood components to the donor. These two methods of platelet collection yield distinctly different amounts of platelets per unit. One platelet concentrate contains approximately 7 × 1010 platelets, whereas one single donor apheresis platelet contains 3 to 6 × 1011 platelets. Pooling of 5 to 8 platelet concentrates from different donors is required to equal the same amount of platelets in an apheresis platelet transfusion. Platelets have a short shelf life (limited to 5 days) and are stored at room temperature and preserved with constant, gentle agitation. The various types of platelet products are listed in Table 442-3, with their approximate volumes, compositions, dosage, and storage periods.
Table 442-3. Platelet Products
 INDICATIONS
INDICATIONS
Normal peripheral blood platelet counts vary in all age groups from 150,000 to 450,000 per μl. The threshold to transfuse platelets in premature neonates is higher than in other age groups, given the risk of intracranial/ventricular hemorrhage (ICH/IVH). When the platelet count falls below 50,000/μl, there is a clinically significant risk of an IVH (particularly in infants who weigh less than 1.5 kg at birth).36 However, prophylactic platelet infusion is controversial, because although this procedure can increase platelet counts and shorten bleeding times, it has not been shown that it reduces the incidence of IVH.37 In contrast, neonates, children, and adolescents can tolerate platelet counts as low as 10,000/μl without the risk of major bleeding. The most common rationale for transfusions is preventing potential bleeding.38 For stable patients, recent studies support a platelet threshold of 10,000/μl when there are no coexisting conditions.39-41 Currently, the same thresholds are used for children/adolescents who have thrombocytopenia secondary to chemotherapy. For children and adults with fever, active bleeding, or coexisting coagulation defects, a platelet count of 20,000/μl is considered the threshold at which to transfuse. 
 ORDERING
ORDERING
When possible, platelets should be ABO and Rh matched, as this secures the best response and minimizes the potential for red cell hemolysis. 
For the neonate and small child, incompatible platelet products, whether apheresis or a platelet concentrate, should be volume reduced to eliminate most of the incompatible plasma. However, the process of volume reduction decreases the number and optimal functioning of platelets and shortens their expiration to 4 hours. Consequently, volume reduction is not routinely recommended for older children or adult patients who receive ABO-mismatched platelet products. 
 DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
The customary dose for a platelet concentrate is 10 ml/kg, which typically yields approximately 10 × 109 cells. Platelets obtained from a single donor may contain a slightly lower dose than the platelet concentrate. Some facilities categorize platelet apheresis products into patient subsets by weight and are ordered as “quarters” or “halves.” Alternatively, a 70-kg adult body surface area (BSA) of 1.7 m2 can be used to estimate the BSA of a neonate or child to approximate the appropriate pediatric dose. When a patient is suspected of being platelet refractory, the clinician must anticipate the time required (up to several hours or even days) to obtain and prepare either crossmatched or HLA-matched platelets. 
PLASMA PRODUCTS
 COMPOSITION AND VOLUME
COMPOSITION AND VOLUME
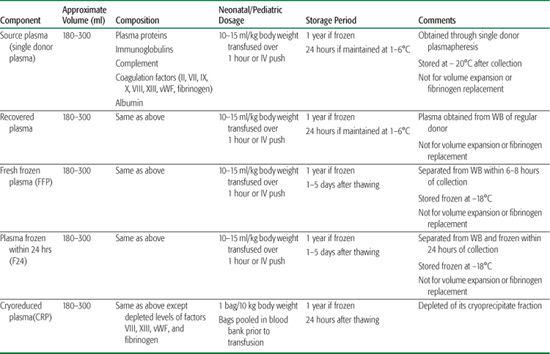
The aqueous, acellular portion of whole blood is known as plasma. Albumin is the most abundant of the plasma proteins. Other plasma proteins include complement, predominantly C3, in addition to enzymes, transport molecules, immunoglobulins (gamma-globulins), and coagulation factors. Coagulation factors in plasma include (1) fibrinogen; (2) factor XIII; (3) von Willebrand factor; (4) factor VIII, primarily bound to its carrier protein vWF (∼ 100 ng/ml); and (5) vitamin K–dependent coagulation factors II, VII, IX, X.1Plasma products are mainly produced from whole blood and less frequently from plasma-pheresis collections. The designation of a plasma product as either fresh frozen plasma (FFP) or F24 plasma is determined by the time after collection to the time of freezing. FFP is frozen within 6 to 8 hours of collection, while F24 is frozen within 24 hours after collection. FFP and F24 are virtually equivalent. Another FDA-approved plasma product is cryo-reduced plasma (CRP), also known as cryosupernatant, which is depleted of its cryoprecipitate fraction. Table 442-4 provides a list of plasma-derived products, with their appropriate volumes, composition, and storage periods.
Table 442-4. Plasma-Derived Products1
 INDICATIONS
INDICATIONS
Specific indications for FFP and F24 include (1) bleeding diatheses associated with acquired coagulation factor deficits, such as end stage liver disease, massive transfusion,49 and disseminated intravascular coagulation (DIC); (2) the rapid reversal of warfarin effect; (3) plasma infusion or exchange for thrombocytopenic purpura (TTP); (4) congenital coagulation defects, except when specific factor therapy is available; and (5) C1-esterase inhibitor deficiency. 
 ORDERING
ORDERING
Despite a lack of formal compatibility testing for plasma, the ordering physician must be aware of isohemagglutinins that can cause hemolysis and accordingly must order ABO-compatible plasma for the recipient. Nevertheless, when a recipient’s ABO type is unknown prior to a plasma infusion, AB plasma, lacking in isohemagglutinins, may be administered. Rh alloimmunization rarely results from an Rh mismatch of plasma products, because there are few RBCs in plasma.
 DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
Dosing of both FFP and F24 is uniform (10–20 ml/kg) among neonates, children, and adults. It typically yields a 30% increment in coagulation factor concentrations. Nonetheless, a clinically significant coagulopathy may require multiple doses of plasma to correct this state. The need to thaw either of these products, which can take 20 to 40 minutes to complete, requires advance notification. Rapid infusion of plasma products is acceptable, and doses may be administered repeatedly, depending on the half-life of the factor deficiency being treated.
CRYOPRECIPITATE
 COMPOSITION AND VOLUME
COMPOSITION AND VOLUME
Cryoprecipitate is an insoluble precipitate that is formed by thawing FFP and then refreezing it in 10 to 15 ml of plasma within 1 hour. This processing produces a plasma-based product containing the highest concentrations of factor VIII (80–150 U/unit), vWF (100–150 U/unit), fibrinogen (∼250 mg/unit), factor XIII (150–250 U/unit) and fibronectin ∼2 mg/ml. Cryoprecipitate can be stored at temperatures less than or equal to –18°C and can be maintained for up to 1 year.
 INDICATIONS
INDICATIONS
The development of safer and more specific factor concentrates has limited the indications for cryoprecipitate primarily to fibrinogen replacement, owing to its high fibrinogen content. The etiology of such a deficiency may be congenital or acquired, namely (1) dysfibrinogenemia; (2) DIC; (3) orthotopic liver transplantation; and (4) poststreptokinase therapy, which may cause hyperfibrinogenolysis. It is also effective in the rare patient with factor XIII deficiency. With the advent of heat-treated, plasma-derived von Willebrand–containing factor products and recombinant factor VIII products, cryoprecipitate no longer has a therapeutic role in treating von Willebrand disease and hemophilia A in the United States. See eTable 442.5  (online text) for more detailed indications for cryoprecipitate.
(online text) for more detailed indications for cryoprecipitate.
 ORDERING
ORDERING
Cryoprecipitate units have a small volume (10–30 ml) in contrast to other plasma products, pRBCs, and apheresis platelets. As a result, the isohemagglutinins anti-A and anti-B are present in small quantities. Similarly to plasma products, compatibility testing is not absolutely required for cryoprecipitate, but it is recommended by the AABB standards for the pediatric patient.
 DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
DOSAGE, BLOOD BANK PREPARATION, ADMINISTRATION
The dose of cryoprecipitate required depends on the clinical necessity. In both children and adults, a 1 unit/10 kg dose will increase the fibrinogen level by 60 to 100 mg/dl. However, in a neonate, a single unit dose will increase the fibrinogen level by more than 100 mg/dl. The dosing frequency varies, from once every 8 to 12 hours to up to several days, depending on the cause of the hypofibrinogenemia. Cryo-precipitate is prepared in the blood bank by thawing and pooling several units, which are then issued as one unit. As with other frozen plasma products, component preparation can take 20 to 30 minutes. Cryoprecipitate can be administered by rapid infusion or slowly over 2 to 4 hours, depending on the clinical indication. 
SPECIAL PROCESSING/TESTING TO PREVENT ADVERSE REACTIONS
Leukoreduction, CMV serological testing, gamma-irradiation, plasma volume reduction, and washing of cellular products (pRBCs and platelets) are specialized processes performed to prevent specific transfusion complications. See online text for further details.
TRANSFUSION REACTIONS AND MANAGEMENT
Transfusion reactions are categorized as noninfectious versus infectious complications of trans-fusion (eTable 442.9  ; see online text). Acute hemolytic transfusion reactions (AHTRs) are noninfectious reactions that result from the transfusion of ABO-incompatible red cells. In acute hemolytic reactions, intravascular hemolysis results from IgM anti-A or anti-B reacting with their cognate antigen on the donor red cells. Intravascular hemolysis can also occur by passive IgM anti-A or anti-B transfer of incompatible donor plasma that reacts with the recipient’s red cells, which carry the cognate antigen. The release of free hemoglobin into the intravascular space activates the coagulation cascade and releases vasoactive amines, resulting in fever, chills, nausea and vomiting, chest or back pain, and anxiety. As a result, laboratory tests may reveal hemoglobin in the urine and low haptoglobin in the serum. The severity of the reaction can be mitigated by the timely cessation of the transfusion. However, the infusion of a large volume of incompatible blood or plasma can result in acute renal failure, shock, disseminated intravascular coagulation (DIC), and death. Acute intravascular hemolysis can be mediated by a nonimmune cause such as physical or thermal injury to donor RBCs during storage (inappropriate temperature storage or mixing of hypotonic fluid with RBCs) or transfusion method (red cells being pressurized through a fine-gauge needle).
; see online text). Acute hemolytic transfusion reactions (AHTRs) are noninfectious reactions that result from the transfusion of ABO-incompatible red cells. In acute hemolytic reactions, intravascular hemolysis results from IgM anti-A or anti-B reacting with their cognate antigen on the donor red cells. Intravascular hemolysis can also occur by passive IgM anti-A or anti-B transfer of incompatible donor plasma that reacts with the recipient’s red cells, which carry the cognate antigen. The release of free hemoglobin into the intravascular space activates the coagulation cascade and releases vasoactive amines, resulting in fever, chills, nausea and vomiting, chest or back pain, and anxiety. As a result, laboratory tests may reveal hemoglobin in the urine and low haptoglobin in the serum. The severity of the reaction can be mitigated by the timely cessation of the transfusion. However, the infusion of a large volume of incompatible blood or plasma can result in acute renal failure, shock, disseminated intravascular coagulation (DIC), and death. Acute intravascular hemolysis can be mediated by a nonimmune cause such as physical or thermal injury to donor RBCs during storage (inappropriate temperature storage or mixing of hypotonic fluid with RBCs) or transfusion method (red cells being pressurized through a fine-gauge needle).
Delayed hemolytic transfusion reactions (DHTRs) can occur 3 to 10 days after a red cell transfusion, owing to non-ABO antigens. These IgG-mediated reactions can cause severe hemolysis. A DHTR can occur in a previously sensitized recipient, whose incompatible minor RBC antigens go undetected during routine antibody screening and produce an anamnestic antibody response. Detection of a DHTR may be delayed, as recipients are initially asymptomatic. However, they subsequently develop hyperbilirubinemia, do not achieve the expected transfusion outcome, or merely have an unexplained decline in hemoglobin level 1 to 2 weeks post-transfusion. Confirmation of a DHTR is made with a positive direct antiglobulin test (DAT) and a potentially positive antibody screen. Thereafter, antibody specificity must be identified and future pRBC transfusions should be devoid of that specific RBC antigen.
Transfusion-related acute lung injury (TRALI) is a noninfectious complication of transfusion and is currently the leading cause of transfusion-related deaths reported to the FDA.61 As defined by the National Heart, Lung and Blood Institute (NHLBI), TRALI is a “new acute lung injury (ALI) that develops with a clear temporal relationship to transfusion, in patients with or without alternate risk factors for ALI.” Contrastingly, the Canadian Consensus Conference on TRALI in 2004 defined it as “a new episode of ALI that occurs during or within 6 hours of a completed transfusion which is not temporally related to a competing etiology for ALI.” A diagnosis of TRALI is confirmed by clinical and radiographic findings. Recipients may exhibit shortness of breath resulting from noncardiogenic pulmonary edema, fever, and hypotension. At present, the pathogenesis, treatment, and prevention of TRALI are not well understood.
Transfusion-associated circulatory overload (TACO) is another noninfectious complication that can result from transfusing excessive fluid in a disproportionate time. Patients at greatest risk for developing TACO are those with underlying cardiovascular disease. For febrile, nonhemolytic transfusion reactions (FNHTRs) and allergic/anaphylactic transfusion reactions, see the discussion in the online text.
INFECTIOUS COMPLICATIONS OF TRANSFUSION
Infectious organisms transmissible in blood include viruses, bacteria, parasites, spirochetes, and prions (eTable 442.10  in online text). The three most common transmitted viral pathogens that have caused significant morbidity and mortality are HIV, hepatitis C (HCV), and hepatitis B (HBV). Significant advances in safety/testing, such as nucleic acid testing (NAT), have greatly reduced the risk of disease transmission in the United States. See eTable 442.11
in online text). The three most common transmitted viral pathogens that have caused significant morbidity and mortality are HIV, hepatitis C (HCV), and hepatitis B (HBV). Significant advances in safety/testing, such as nucleic acid testing (NAT), have greatly reduced the risk of disease transmission in the United States. See eTable 442.11  (online text) for a list of transfusion-transmitted viral agents.
(online text) for a list of transfusion-transmitted viral agents. 
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


