Fig. 13.1
Correlation of T3, T4 and TSH values with thyroid disorders observed during screening
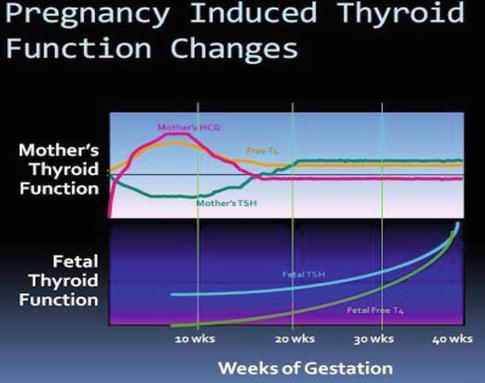
Fig. 13.2
Changes in maternal and foetal thyroid function during pregnancy
Interestingly, the secretion of T4 and T3 is not similar for all pregnant women [4]. Approximately a third of women experience relative hypothyroxinaemia, preferential T3 secretion and higher, albeit normal, serum thyrotropin levels. Thus, there may be considerable variability in thyroidal adjustments during normal pregnancy.
Thyrotropin, or thyroid-stimulating hormone (TSH), currently plays central role in screening and diagnosis of many thyroid disorders. Serum thyrotropin levels in early pregnancy decrease because of thyroid stimulation from the weak TSH effects of human chorionic gonadotropin (hCG) [5]. TSH does not cross the placenta. At the same time, hCG serum levels are maximal for the first 12 weeks; free thyroxin levels increase to suppress the pituitary thyrotropin secretion. Accordingly, thyrotropin-releasing hormone (TRH) is undetectable in maternal serum. Fetal serum TRH is detectable beginning at mid-pregnancy, but does not increase.
Throughout pregnancy, maternal thyroxine transferred to the fetus [6]. Maternal thyroxine is important for normal fetal brain development, especially prior to development of fetal thyroid gland function [7]. And even though the fetal thyroid gland begins concentrating iodine and synthesizing thyroid hormone after 12 weeks, maternal thyroxine contribution remains important. In fact, maternal thyroxine accounts for 30 % of thyroxine in fetal serum at term [8] (Fig. 13.3).
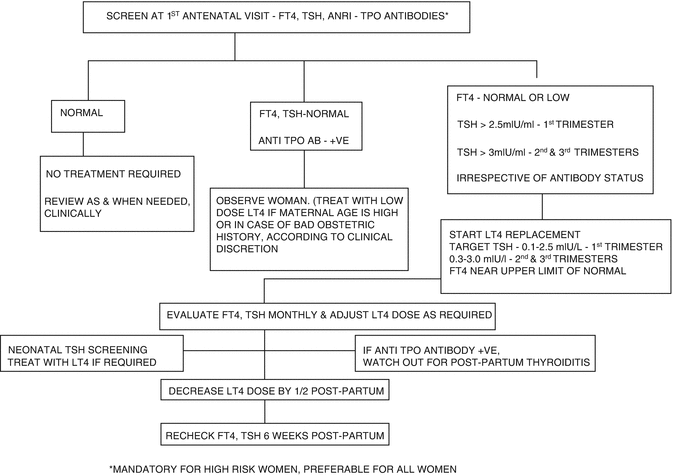

Fig. 13.3
Flowchart depicting treatment based on screening
TSH: Levels Are Trimester-Dependent
Ranges [1]
0.2–2.5 mlU/L in the first trimester
0.3–3.0 mlU/L in the second trimester
Up to 3.5 mlU/L in the third trimester
Free T4 varies as albumin and T4-binding globulin change.
Numerous hormonal changes and metabolic demands occur during pregnancy, resulting in profound and complex effects on thyroid function.
Table 13.1 summarizes the main physiologic changes that occur during a normal pregnancy and that relate to thyroid function or thyroid function testing. These changes are discussed below.
Table 13.1
Factors affecting thyroid physiology during normal pregnancy
Physiologic change
Thyroid-related consequences
Increased renal iodine clearance
Increased 24-h RAIU
Decreased plasma iodine and placental iodine transport to the fetus
In iodine-deficient women, decreased T4, increased TSH and goitre formation
Increased O2 consumption by fetoplacental unit, gravid uterus and mother
Increased BMR
First-trimester increase in hCG
Increased free T4 and T3 and decreased basal TSH (partial blunting of the pituitary-thyroid axis)
Increased serum TBG
Increased total T4 and T3
Increased plasma volume
Increased T4 and T3 pool size
Inner-ring deiodination of T4 and T3 by placenta
Accelerated rates of T4 and T3 degradation and production
Hyperthyroidism
Hyperthyroidism occurs in about 0.2–0.4 % of all pregnancies. Most cases are due to Graves’ disease although less common causes (e.g. toxic nodules and thyroiditis) may be seen [9]. Clinical assessment alone may occasionally be inadequate in differentiating hyperthyroidism from the hyperdynamic state of pregnancy. Distinctive clinical features of Graves’ disease include the presence of ophthalmopathy, diffuse goitre and pretibial myxoedema. Also, hyperthyroidism must be distinguished from gestational transient thyrotoxicosis, a self-limiting hyperthyroid state due to the thyroid stimulatory effects of beta-hCG. This distinction is important since the latter condition is typically mild and will not usually require specific antithyroid treatment. Hyperthyroidism due to Graves’ disease may worsen in the first trimester of pregnancy, remit in later pregnancy and subsequently relapse in the postpartum (Fig. 13.4).
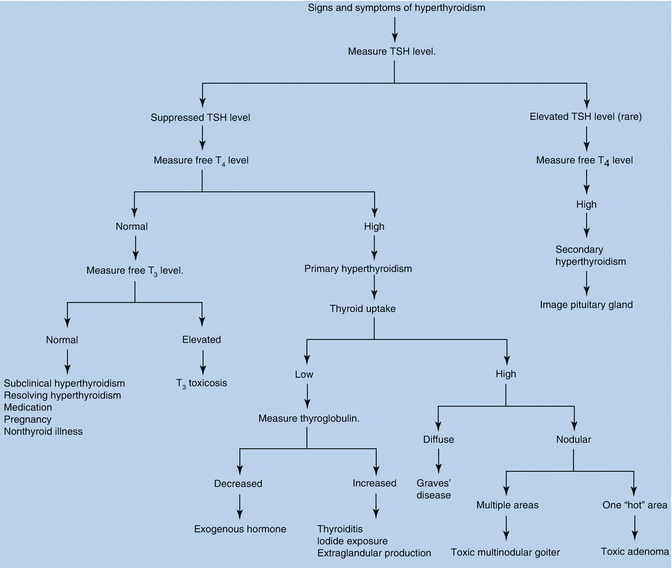

Fig. 13.4
Signs and symptoms of hyperthyroidism
Laboratory confirmation is by a markedly depressed TSH level along with an elevated serum-free T4 (fT4) level. Rarely, hyperthyroidism is caused by abnormally high serum triiodothyronine (T3) levels – the so-called T3 thyrotoxicosis (Fig. 13.5).
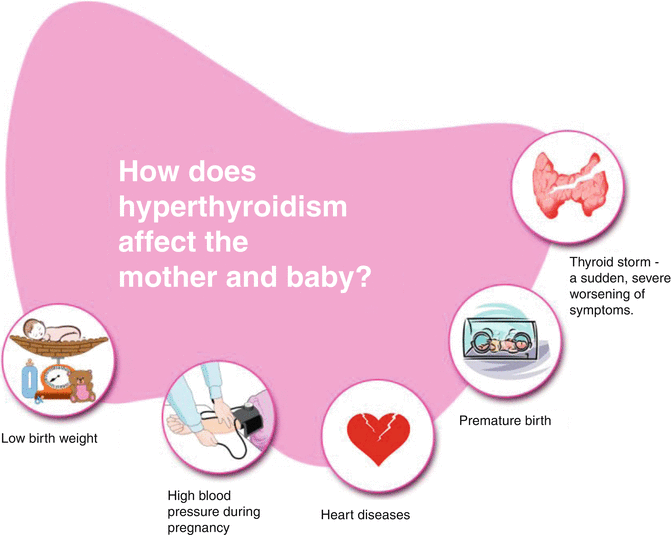

Fig. 13.5
Effect of hyperthyroidism in mother and baby
Risks of Hyperthyroidism on Fetal and Maternal Well-Being
Uncontrolled hyperthyroidism in pregnancy is associated with an increased risk of severe pre-eclampsia and up to a fourfold increased risk of low-birth-weight deliveries. Some of these unfavourable outcomes are more marked in women who are diagnosed for the first time in pregnancy.
Uncontrolled and inadequately treated maternal hyperthyroidism may also result in fetal and neonatal hyperthyroidism [10] due to the transplacental transfer of stimulatory TSH-receptor antibodies (TRAbs) [11]. Clinical neonatal hyperthyroidism occurs in about 1 % of infants born to mothers with Graves’ disease. Rarely neonatal hypothyroidism may also be observed in the infants of mothers with Graves’ hyperthyroidism. This may result from transplacental transfer of circulating maternal anti-thyroid drugs and pituitary-thyroid axis suppression from transfer of maternal thyroxine.
Poorly controlled hyperthyroidism during pregnancy is associated with the following:
Maternal:
Investigations
Serum TSH can exclude primary thyrotoxicosis. Confirm diagnosis with free T4 levels. If TSH is suppressed but free T4 levels are normal, then, if not previously supplied, free T3 level is necessary (T3 toxicosis occurs in 5 % of patients). Previously successfully treated Graves’ disease is not associated with abnormal TFTs during pregnancy [13]. It is important to remember that the ranges of TSH, T3 and T4 are different in pregnancy [14]:
Pre-pregnancy Hyperthyroidism Counselling [15]
This should be offered to all women. The main points about which to raise awareness are:
General pregnancy and pre-conception advice to all women – e.g. folic acid.
Pre-conception patients may be offered definitive therapy – e.g. ablation with radiotherapy (ideally, the patient should not conceive until 3–6 months later, once the levothyroxine dose has been optimized).
Monitor thyroid-stimulating hormone (TSH) and thyrotropin receptor-stimulating antibodies (TRAb) – they gradually disappear following surgery, whilst with radiotherapy they rise and then usually fall after 12 months.
Thus, surgery is usually the therapy of choice in women planning to become pregnant.
Following definitive therapy, levothyroxine dosage may need to be increased early in pregnancy (increased T4 requirement).
If definitive therapy is not to be considered, then the importance of adhering to medication must be stressed, as there is risk of multiple complications, both maternal and fetal.
Reports from America concerning liver toxicity are being investigated [16].Propylthiouracil is, however, less likely to cross the placenta than carbimazole and has been considered the preferred antithyroid drug. These issues should be discussed with the patient. The safest option may be to use propylthiouracil in early pregnancy, changing to carbimazole in the latter months.
Close follow-up during pregnancy, with TRAb status checked around 24–28 weeks to assess the risk of fetal and/or neonatal hyperthyroidism.
There is a risk of disease worsening during the first trimester or in the early postpartum period; however, note that women may actually have better control of hyperthyroidism during pregnancy.
Antithyroid medication is safe when breast-feeding.
Causes of Relapse of Previously Controlled Hyperthyroidism during Pregnancy
Increase in TRAb in the first trimester.
High levels of human chorionic gonadotropin (hCG) stimulating the thyroid gland.
Impaired drug absorption through vomiting.
Labour, infection and caesarean section may also worsen thyroid control.
Management [17, 18]
Hyperthyroidism during pregnancy can present as hyperemesis gravidarum or as thyroid storm – always check the TFTs. These women need urgent admission to hospital [19].
Note: Hyperemesis gravidarum is associated with abnormal TFTs which improve once it settles. Control is particularly important as the pregnancy progresses, especially in the third trimester. This is the result of suppression of the fetal pituitary-thyroid axis from maternal transfer of thyroxine when hyperthyroidism is poorly controlled.
Pregnant Mothers with a New Diagnosis of Hyperthyroidism.
All pregnant women should be referred urgently for assessment of a new diagnosis.
Treatment of All Cases of Hyperthyroidism during Pregnancy (New Diagnoses or Worsening of Previously Controlled Hyperthyroidism)
Antithyroid drugs are the first line for all.
Radioiodine is contraindicated.
Surgery is only where absolutely necessary and requires the patient to be rendered euthyroid with drugs to begin with.
All cases should be discussed with a specialist.
This needs to be urgently referred if adrenergic symptoms are present which may require treatment.
Adrenergic symptoms can be treated with short courses of beta-blockers – e.g. propranolol. Use beyond a few weeks may adversely affect the fetus and is not advised.
Antithyroid drugs:
Propylthiouracil may cross the placenta less readily than carbimazole (which has, on rare occasions, been associated with teratogenic affects) and it is the first choice in pregnancy and breast-feeding. However, liver toxicity has been recently reported (see section “Pre-pregnancy hyperthyroidism counselling” above). Current opinion favours using propylthiouracil in early pregnancy and carbimazole in later months [17].
Rarely, carbimazole has been associated with teratogenic affects.
However, in some countries, carbimazole may be the only choice available and the risks of not treating maternal hyperthyroidism will far outweigh those of potential teratogenicity.
The aim is to keep the thyroid hormones in the upper third of the reference range. Once this is achieved, then the dose of propylthiouracil is decreased to prevent effects on neonatal thyroid function (may produce neonatal hypothyroidism). A similar strategy is used in Graves’ disease presenting during pregnancy.
Block and replace regimen is not recommended and medications need to continue into labour – albeit at a lower dose.
Remember that antithyroid drugs may cause neonatal hypothyroidism – thus, a minimal dose required should be used and thyroid hormones should be kept within the upper third of the normal range.
All monitoring of pregnant women should take place in secondary care but a full TFT profile should be sent from primary care. Monitoring usually involves the following: Measure TFTs every 4–6 weeks.
Serial fetal ultrasonography (looking for intrauterine growth restriction, hydrops fetalis, advanced bone age, goitre, tachycardia and heart failure).
Check TRAb.
Thyrotoxicosis
The overwhelming cause of thyrotoxicosis in pregnancy is Graves’ disease, an organ-specific autoimmune process usually associated with thyroid-stimulating antibodies. Such antibody activity declines during pregnancy, and it may become undetectable in the third trimester [20].
Treatment of Thyrotoxicosis during Pregnancy
Thyrotoxicosis during pregnancy can nearly always be controlled by thionamide drugs. Some clinicians prefer propylthiouracil (PTU) because it partially inhibits the conversion of T4–T3 and crosses the placenta less readily than methimazole. Although not definitely proven, methimazole use in early pregnancy has been associated with a rare methimazole embryopathy characterized by oesophageal or choanal atresia as well as aplasia cutis, which is a congenital skin defect [21, 22]. Although these malformations are uncommon in women treated with methimazole, and despite the lack of epidemiological studies that PTU is safer, PTU is still preferred thionamide in the United States [23].
Transient leukopenia can be documented in up to 10 % of women taking antithyroid drugs but does not require cessation of therapy. In 0.3–0.4 %, agranulocytosis develops suddenly and mandates discontinuance of the drug. It is not dose related, and because of its acute onset, serial leukocyte count during therapy is not helpful. Thus, if fever or sore throat develops, women are instructed to discontinue medication immediately and report for a complete blood count [23]. Hepatotoxicity is another potentially serious side effect that occurs in 0.1–0.2 %. Approximately 20 % of patients treated with PTU develop antineutrophil cytoplasmic antibody (ANCA), but only a small percentage of these go on to develop serious vasculitis [24]. Finally, although thionamides have a potential to cause serious fetal complications, these are uncommon. In some cases, thionamides may even be therapeutic, because thyrotropin receptor antibodies cross the placenta and can stimulate the fetal thyroid gland to cause thyrotoxicosis and goitre.
The initial propylthiouracil dose is empirical. For nonpregnant patients, the American Thyroid Association recommends an initial daily dose of 100–600 mg for PTU or 10–40 mg for methimazole [25]. With a PTU dose that averaged 600 mg daily, only half of women had remission, and in these, the dose was decreased to less than 300 mg daily within 8 weeks. In a third, however, it was necessary to increase the dose. Serum free T4 is considered a better indicator of thyroid status than TSH during first 2–3 months of treatment for hyperthyroidism [26].
Subtotal thyroidectomy can be performed after thyrotoxicosis is medically controlled. This seldom is done during pregnancy but may be appropriate for the very few women who cannot adhere to medical treatment or in whom drug therapy proves toxic [27]. Ablation with therapeutic radioactive iodine is contraindicated during pregnancy. Therapeutic doses for maternal thyroid disease may also cause fetal thyroid gland destruction. Thus, when given unintentionally, most clinicians recommend abortion. Any exposed infant must be carefully evaluated for hypothyroidism [28]. The incidence of fetal hypothyroidism depends on gestational age and radioactive dose [29]. There is no evidence that therapeutic radioiodine given before pregnancy causes fetal anomalies if enough time has passed to allow radiation effects to dissipate and the woman is euthyroid [30, 31]. The International Commission on Radiological Protection has recommended that women avoid pregnancy for 6 months after radioablative therapy [23].
Thyroid Storm
Only about 1–2 % of women with hyperthyroidism who receive thionamide experience thyroid storm – but it is a devastating complication [32].
Thyroid storm is a rare, life-threatening endocrinologic emergency that can lead to cardiac arrest and death. A total of 20–30 % of all cases are fatal [33]. Maternal mortality for this condition is currently approximately 3 %. Pregnant women with hyperthyroidism are at increased risk for spontaneous pregnancy loss, congestive heart failure, thyroid storm, preterm birth, pre-eclampsia, fetal growth restriction and associated with increased perinatal morbidity and mortality [34]. Patients can have a wide range of signs and symptoms. The tachycardia is often out of proportion to the hyperthermia. Blood pressure is commonly normal, although a widened pulse pressure is common. Patients with thyroid storm usually appear confused and disoriented. Thyroid storm can be precipitated by surgery, infection, trauma or labour and delivery [35, 36]. Patients with thyroid storm require assessment and management in an intensive care unit where they can be monitored for cardiac status, fluid and electrolyte balance and control of hyperthermia [37]. The underlying cause of thyroid storm must be identified and treated.
Clinical Features
Hyperthermia
Nausea
Abdominal pain
Vomiting
Severe agitation
Diaphoresis
Dehydration
Tachycardia
Congestive heart failure
Arrhythmia
Confusion
Cardiovascular collapse
Malignant exophthalmos
Management (Table 13.2)
Table 13.2
Three-step treatment of thyroid storm
Goal | Treatment | Effect | |
|---|---|---|---|
Step 1 | Block peripheral effect of thyroid hormone | Provide continuous intravenous infusion of β-blocking agent | Slows heart rate, increases diastolic filling and decreases tremor |
Step 2 | Stop the production of thyroid hormone | Provide antithyroid medication (propylthiouracil or methimazole) and dexamethasone | Antithyroids decrease synthesis of thyroid hormone in the thyroid. Propylthiouracil slows conversion of T4 to T3 in periphery. Dexamethasone decreases conversion of T4 to T3 in periphery |
Step 3 | Inhibit hormone release | Give iodide 1–2 h after antithyroid medication | Decreases release of thyroid hormone from thyroid |
Patients with thyroid storm should be treated in an ICU setting for close monitoring of vital signs and for access to invasive monitoring and inotropic support, if necessary. Initial stabilization and management of systemic decompensation are as follows:
If needed, immediately provide supplemental oxygen, ventilatory support and intravenous fluids. Dextrose solutions are the preferred intravenous fluids to cope with continuously high metabolic demand.
Correct electrolyte abnormalities.
Treat cardiac arrhythmia, if necessary.
Aggressively control hyperthermia by applying ice packs and cooling blankets and by administering acetaminophen (15 mg/kg orally or rectally every 4 h).
Promptly administer antiadrenergic drugs (e.g. propranolol) to minimize sympathomimetic symptoms.
Correct the hyperthyroid state. Administer antithyroid medications to block further synthesis of thyroid hormones (THs).
High-dose propylthiouracil (PTU) is preferred because of its early onset of action and capacity to inhibit peripheral conversion of T4–T3. The US Food and Drug Administration (FDA) had added a boxed warning, the strongest warning issued by the FDA, to the prescribing information for PTU.
The boxed warning emphasizes the risk for severe liver injury and acute liver failure, some of which have been fatal. The boxed warning also states that PTU should be reserved for use in those who cannot tolerate other treatments such as methimazole, radioactive iodine or surgery.
The decision to include a boxed warning was based on the FDA’s review of postmarketing safety reports and meetings held with the American Thyroid Association, the National Institute of Child Health and Human Development and the paediatric endocrine clinical community.
The FDA has identified 32 cases (22 adult and 10 paediatric) of serious liver injury associated with PTU. Among adults, 12 deaths and 5 liver transplants occurred; among the paediatric patients, 1 death and 6 liver transplants occurred. PTU is indicated for hyperthyroidism due to Graves’ disease. These reports suggest an increased risk for liver toxicity with PTU compared with methimazole. Serious liver injury has been identified with methimazole in five cases (three resulting in death).
PTU is considered as a second-line drug therapy, except in patients who are allergic or intolerant to methimazole, or for women who are in the first trimester of pregnancy. Rare cases of embryopathy, including aplasia cutis, have been reported with methimazole during pregnancy. The FDA recommends the following criteria be considered for prescribing PTU [38]:
Reserve PTU use during first trimester of pregnancy or in patients who are allergic to or intolerant of methimazole.
Closely monitor PTU therapy for signs and symptoms of liver injury, especially during the first 6 months after initiation of therapy.
For suspected liver injury, promptly discontinue PTU therapy and evaluate for evidence of liver injury and provide supportive care.
PTU should not be used in paediatric patients unless the patient is allergic to or intolerant of methimazole, and no other treatment options are available.
Counsel patients to promptly contact their health-care provider for the following signs or symptoms: fatigue, weakness, vague abdominal pain, loss of appetite, itching, easy bruising or yellowing of the eyes or skin.
Administer iodine compounds (Lugol’s iodine or potassium iodide) orally or via a nasogastric tube to block the release of THs (at least 1 h after starting antithyroid drug therapy). If available, intravenous radiocontrast dyes such as ipodate and iopanoate can be effective in this regard. These agents are particularly effective at preventing peripheral conversion of T4–T3.
Administer glucocorticoids to decrease peripheral conversion of T4–T3. This may also be useful in preventing relative adrenal insufficiency due to hyperthyroidism.
Treat the underlying condition, if any, that precipitated thyroid storm and exclude comorbidities such as diabetic ketoacidosis and adrenal insufficiency. Infection should be treated with antibiotics.
Rarely, as a life-saving measure, plasmapheresis has been used to treat thyroid storm in adults [33].
Iodine preparations should be discontinued once the acute phase resolves and the patient becomes afebrile with normalization of cardiac and neurological status. Glucocorticoids should be weaned and stopped and the dose of thioamides adjusted to maintain thyroid function in the normal range. Beta-blockers may be discontinued once thyroid function normalizes.
If the patient is given PTU during treatment of thyroid storm, this should be switched to methimazole at the time of discharge unless methimazole is contraindicated. If there is a contraindication for the use of methimazole, alternative methods to treat hyperthyroidism should be considered after discharge, such as radioactive iodine or surgery.
Aims of Therapy
Therapy is aimed (1) ameliorating hyperadrenergic effects of thyroid hormone (TH) on peripheral tissues with use of beta-blockers (e.g. propranolol, labetalol); (2) decreasing further synthesis of THs with antithyroid medications (e.g. propylthiouracil [PTU], methimazole); (3) decreasing hormonal release from the thyroid, using iodides; and (4) preventing further TH secretion and peripheral conversion of T4 to T3, using glucocorticoids or iodinated radiocontrast dyes when available.
Prevention
The gold standard of treatment of thyroid storm is primary prevention. Prevention of thyroid storm requires careful control and management of the hyperthyroidism. Standard treatment options for Graves’ disease include therapy with radioactive iodine, ATDs and thyroid surgery [25]. However, pregnancy limits these treatment options. Because of possible destruction of the thyroid gland in the fetus, radioactive iodine cannot be given, and surgery is avoided because of the increased risk for miscarriage or preterm delivery.
As a result, the standard treatment during pregnancy is the use of ATDs to inhibit the biosynthesis of thyroid hormones. Because of the immunosuppressive effect of pregnancy, ATDs can be given in lower doses in pregnant patients than in nonpregnant patients. Every attempt should be made to treat with the lowest possible effective dose of ATDs because these drugs can cross the placenta, enter the fetal circulation and affect the thyroid gland of the fetus.
Even though propylthiouracil is the drug of choice during pregnancy, it is not given without careful observation, because it results in drug reactions in up to 5 % of treated patients. These reactions include fever, rash, urticaria, arthralgias and leukopenia. A rare adverse effect, agranulocytosis, an acute condition distinguished by a deficit or absolute lack of granulocytes, usually is manifested by fever and sore throat. If fever and sore throat occur, a complete blood cell count should be done, and if agranulocytosis is diagnosed, treatment with thiopropyluracil should be stopped [39].
The starting dose is typically 300–450 mg/day divided into 3 doses. If methimazole is used, the starting dose is 20 mg twice a day. Results of laboratory tests should be monitored carefully, and once a patient becomes euthyroid, the dose can be tapered gradually. Many patients need only 50 mg/day, and some patients may not need any medication by the third trimester; however, the dosage may vary from 50 to 200 mg of propylthiouracil every 8 h or methimazole 10–60 mg/day, depending on the patient’s signs and symptoms and laboratory values [40, 41]. Biochemically, the aim is to keep the serum level of total T4 between 154 and 193 nmol/L (12–15 μg/dL) and the serum level of free T4 within the reference range for the laboratory test used. (These values will vary from one laboratory to another [41].)
Fetal and neonatal hypothyroidism, as well as the occurrence of goitres, may occur from passage of thionamides across the placenta [40]. During the first trimester, transfer of ATDs transplacentally can affect thyroid development in the fetus. Fetal exposure to ATDs can produce hypothyroidism and fetal growth restriction [42].
Methimazole therapy may be associated with aplasia cutis (a localized lesion in the parietal area of the scalp, characterized by congenital absence of the skin, punched-out “ulcer” lesions that usually heal spontaneously) in the offspring of treated women and is another reason that propylthiouracil has become the drug of choice during pregnancy [43]. The therapeutic goal is to control the mother’s hyperthyroidism by using the smallest possible amount of medication, to avoid suppressing the thyroid gland in the fetus [44].
Hyperemesis Gravidarum and Gestational Thyrotoxicosis (Fig. 13.6)
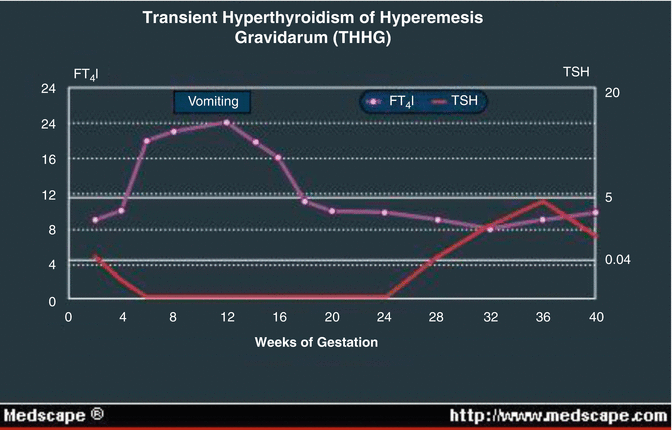
Fig. 13.6
Relationship of hyperemesis to T.S.H.
Hyperemesis gravidarum is associated with abnormal TFTs which improve once it settles. Control is particularly important as the pregnancy progresses, especially in the third trimester. This is the result of suppression of the fetal pituitary-thyroid axis from maternal transfer of thyroxine when hyperthyroidism is poorly controlled.
Subclinical Hyperthyroidism and Pregnancy Outcomes [45]
Subclinical hyperthyroidism has long-term sequelae that include osteoporosis, cardiovascular morbidity and progression to overt thyrotoxicosis or thyroid failure.
Subclinical hyperthyroidism is characterized by circulating thyrotropin (thyroid-stimulating hormone; TSH) levels below the reference range and normal serum thyroid hormone levels [46]. The diagnosis is primarily biochemical and depends on the definition of “normal” TSH levels. In 2002, a panel of experts established the reference range for serum THS levels between 0.45 and 4.5 μU/mL.
Whilst interpreting serum TSH levels, physiologic variations as well as presence of occult thyroid disease should be considered. Several anthropometric variables, including age, gender, race and body mass index (BMI), have a noticeable influence over circulating TSH levels [47–49]. In addition, other factors such as concurrent medication, coexisting pregnancy or concomitant diseases should be considered in order to correctly interpret TSH, thyroxin (T4) and triiodothyronine (T3) status [47].
Additionally, clinicians should consider three important facts whilst interpreting the results of thyroid function tests [50]: (1) TSH secretion follows a circadian rhythm with higher values early in the morning and lowest value in the afternoon, (2) TSH secretion is pulse-regulated and (3) TSH half-life is about 15 min.
A classification system for subclinical hyperthyroidism has been proposed recently, which differentiates between low serum TSH levels (0.1–0.4 μU/mL; Grade I or mild) and suppressed TSH concentration (<0.1 μU/mL; Grade II or severe) [51]. Grade I subclinical hyperthyroidism is three to four times more common than Grade II subclinical hyperthyroidism. The risk of progression from Grade I to overt disease is very low. Conversely, about 2–5 % of the cases of Grade II hyperthyroidism progress to clinical disease per year [51].
In some cases, subclinical hyperthyroidism may present with a normal serum level of free T4 (FT4) whilst the serum T3 level remains above the reference range. This unusual laboratory finding has been called “T3 toxicosis” and may represent the earliest stages of disease, which is normally caused by an autonomously functioning thyroid nodule [46]. All these categories are relevant in clinical practice.
Clinical manifestations of both overt and mild (or subclinical) thyrotoxicosis are similar, but differ in magnitude. Potential complications of untreated subclinical hyperthyroidism are numerous and include weight loss, osteoporosis, atrial fibrillation, embolic events and altered cognition. The most profound consequences of subclinical overactive thyroid dysfunction are observed on the cardiovascular system [52] and the skeleton [53].
2. Severity: In general, patients with Grade I subclinical hyperthyroidism may not be offered treatment. On the other hand, treatment should be strongly considered in high-risk individuals with persistent endogenous Grade II (TSH level <0.1 μU/mL) subclinical hyperthyroidism [51].
There was an increased risk of TSH above 3 muU/mL in women who consumed 200 mcg or more of iodine supplements daily compared with those who consumed less than 100 mcg/day (adjusted odds ratio = 2.5 [95 % confidence interval = 1.2–5.4]). We observed no association between urinary iodine and TSH levels. Pregnant women from the area with the highest median urinary iodine (168 mcg/L) and highest supplement coverage (93 %) showed the lowest values of serum-free thyroxine (geometric mean = 10.09 pmol/L [9.98–10.19]).
Iodine supplement intake in the first half of pregnancy may lead to maternal thyroid dysfunction in iodine-sufficient or mildly iodine-deficient populations.
Hypothyroidism
The most common cause of hypothyroidism in pregnancy is Hashimoto thyroiditis, characterized by glandular destruction by autoantibodies, particularly antithyroid peroxidase antibodies. Clinical identification of hypothyroidism is especially difficult during pregnancy because many of the signs or symptoms are also common to pregnancy itself. Thyroid testing should be performed on symptomatic women or those with a history of thyroid disease [54]. Severe hypothyroidism with pregnancy is uncommon, probably because it is often associated with infertility and increased miscarriage rates [55] (Table 13.3).
Table 13.3
Clinical signs and symptoms of hypothyroidism
Constitutional/general | Fatigue, weight gain, cold intolerance, hoarseness, periorbital oedema |
Cardiovascular | Bradycardia, diastolic hypertension. Peripheral oedema, hyperlipidaemia, pericardial effusions |
Pulmonary | Dyspnoea, pleural effusions |
Gastrointestinal | Constipation |
Genitourinary | Decreased glomerular filtration rate, elevated creatinine, infertility, menorrhagia |
Neurological | Poor memory. Difficulty concentrating. Ataxia, muscle weakness. Muscle cramping. Nerve entrapment syndromes (carpal tunnel syndrome), delayed tendon reflex relaxation, paraesthesias, impaired hearing, psychosis |
Dermatological | Dry coarse skin, diffuse alopecia, yellow skin |
Hypothyroidism is common in pregnancy with an estimated prevalence of 2–3 % and 0.3–0.5 % for subclinical and overt hypothyroidism, respectively [56]. Endemic iodine deficiency accounts for most hypothyroidism in pregnant women worldwide whilst chronic autoimmune thyroiditis is the most common cause of hypothyroidism in iodine-sufficient parts of the world [57]. The presentation of hypothyroidism in pregnancy is not always classical and may sometimes be difficult to distinguish from the symptoms of normal pregnancy. A high index of suspicion is therefore required especially in women at risk of thyroid disease, e.g. women with a personal or family history of thyroid disease, goitre or coexisting primary autoimmune disorder like type 1 diabetes.
Risks of Hypothyroidism on Fetal and Maternal Well-Being (Fig. 13.7)
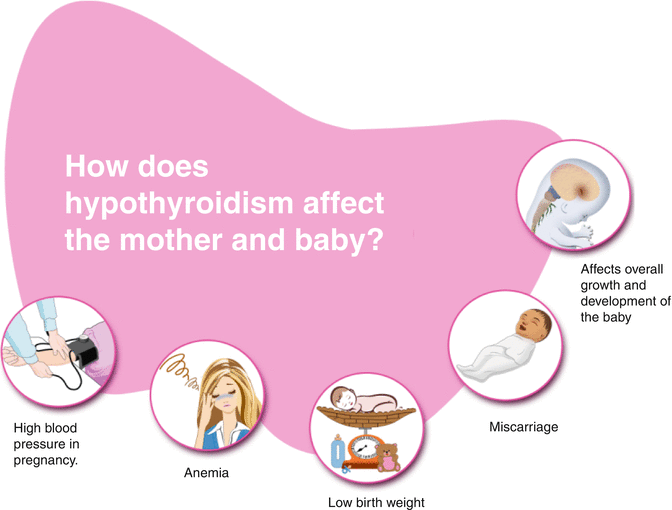
Fig. 13.7
Effect of hypothyroidism on mother and baby
Hypothyroidism is diagnosed by noting a high TSH associated with a subnormal T4 concentration. Subclinical hypothyroidism (SCH) is present when the TSH is high but the T4 level is in the normal range but usually low normal. SCH is the most common form of hypothyroidism in pregnancy and is usually due to progressive thyroid destruction due to autoimmune thyroid disease. Several studies, mostly retrospective, have shown an association between overt hypothyroidism and adverse fetal and obstetric outcomes [58]. Maternal complications such as miscarriages, anaemia in pregnancy, pre-eclampsia, abruptio placenta and postpartum haemorrhage can occur in pregnant women with overt hypothyroidism. Also, the offspring of these mothers can have complications such as premature birth, low birth weight and increased neonatal respiratory distress [59]. Similar complications have been reported in mothers with subclinical hypothyroidism. A threefold risk of abruptio placenta and a twofold risk of preterm delivery were reported in mothers with subclinical hypothyroidism [60]. Another study showed a higher prevalence of subclinical hypothyroidism in women with preterm delivery (before 32 weeks) compared to matched controls delivering at term [61]. An association with adverse obstetrics outcome has also been demonstrated in pregnant women with thyroid autoimmunity independent of thyroid function. Treatment of hypothyroidism reduces the risks of these adverse obstetric and fetal outcomes; a retrospective study of 150 pregnancies showed that treatment of hypothyroidism led to reduced rates of abortion and premature delivery. Also, a prospective intervention trial study showed that treatment of euthyroid antibody-positive pregnant women led to fewer rates of miscarriage than nontreated controls [62].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


