Thrombophilic Disorders
INTRODUCTION
Thromboembolic events are rare in childhood; however, neonates are disproportionately affected by thrombosis. The propensity for neonates to clot may be due to several contributing factors. First, neonates have an immature hemostatic system, which generally gives them physiologic thrombophilia. In addition, they are at high risk for sepsis, which leads to thrombosis due to inflammation or disseminated intravascular coagulopathy (DIC). The high use of central catheters, both arterial and venous, in neonates also increases their risk of thrombosis. Although often considered in neonatal thrombosis, the contributing role of inherited thrombophilias in both arterial and venous thrombotic events in this age group remains poorly defined.
Clinicians caring for neonates should be especially aware of clinical thrombotic events common in the neonate, including portal vein thrombosis, renal vein thrombosis, purpura fulminans, and neonatal stroke. The portal vein is a commonly affected anatomic vein, which is attributed to the use of central catheters. Renal vein thromboses have long been recognized as occurring spontaneously in neonates. Neonatal purpura fulminans is a rare condition of dermal microvascular thrombosis associated with DIC and perivascular hemorrhage. This is often associated with inherited thrombophilias, specifically protein C deficiency. Neonatal central nervous system thrombotic events, including cerebral sinovenous thrombosis and neonatal ischemic stroke, are important thrombotic events. These are discussed in detail in another chapter. In addition to familiarity with these sites of thromboses, it is crucial for clinicians to be aware of the different treatments that may be considered for the best long-term outcomes.
VENOUS THROMBI
The majority of neonatal thrombi occur in the venous system, and most are associated with the placement of central venous catheters. Many of these cases are asymptomatic, though catheter-associated thrombi may present with catheter dysfunction. Symptomatic thrombi often present with swelling of the limbs and lower body in the case of inferior vena cava thrombosis versus swelling of the arm, head, and neck seen in superior vena cava thrombosis (otherwise known as SVC syndrome). Due to the frequent use of umbilical venous lines, neonates also may develop portal vein thrombosis, which can lead to hepatic lobar atrophy or portal hypertension. Intracardiac thrombosis may also develop. These thrombi are usually located in the right atrium and are often associated with central venous lines. The most frequent location for spontaneous venous thrombi in neonates is in the renal veins. Infants with renal vein thrombosis may present with macroscopic or microscopic hematuria, thrombocytopenia, or a palpable flank mass.
ARTERIAL THOMBI
Neonatal arterial thrombi outside the central nervous system are almost exclusively due to iatrogenic causes. Rarely, a spontaneous thrombus may develop in the aorta. Femoral artery catheters used for cardiac catheterization and umbilical artery or peripheral arterial catheters used for blood pressure and blood gas monitoring are the main risk factors for arterial thrombi. These cases may be asymptomatic or present with signs of ischemia or organ dysfunction. Involved extremities usually become cool and pale with poor perfusion. If the thrombus occludes the renal artery or mesenteric artery, the infant may develop hypertension with renal failure or necrotizing enterocolitis.
MATERNAL RISK FACTORS
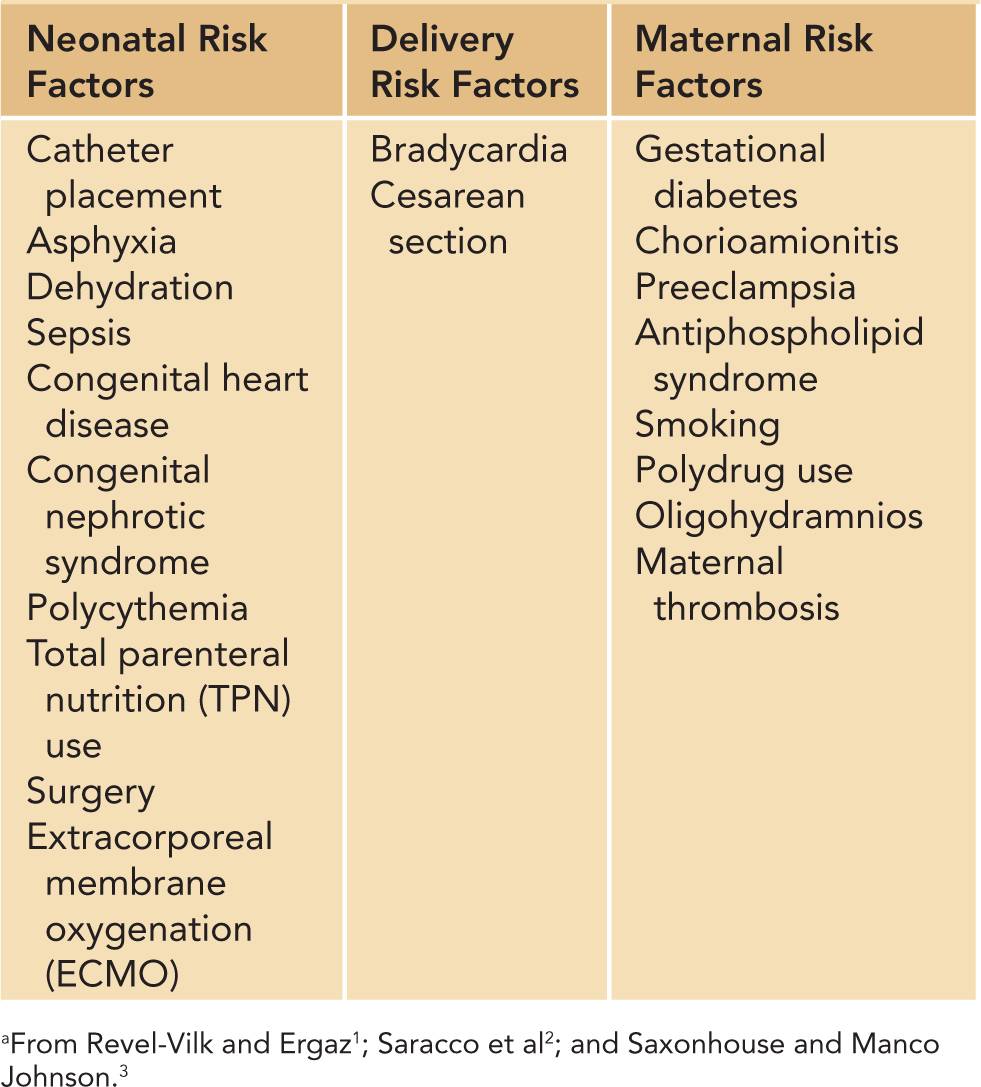
The influence of maternal thrombophilia and prenatal risks on the development of neonatal thrombosis is not well defined. There have been reports of peri- and neonatal infarction associated with thrombosis and infarction of the placenta. Preeclampsia, gestational diabetes, chorioamnionitis, and maternal smoking or drug use have all been associated with increased risk of thrombosis (Table 34-1). Maternal antiphospholipid syndrome (APS) is another rare cause of neonatal thrombosis.
Table 34-1 Acquired Risk Factors for the Development of Neonatal Thrombosisa
INFANT RISK FACTORS
The majority of neonates who develop thrombi have associated risk factors. The most notable risk factor is the presence of a central venous catheter, which is present in approximately 90% of neonates with thrombosis.4,5 In addition, neonatal thrombosis may be the initial presentation of an inherited thrombophilia. Other risk factors include sepsis, asphyxia, heart disease, surgery, dehydration, and nephrotic syndrome (Table 34-1).
Developmental Hemostasis
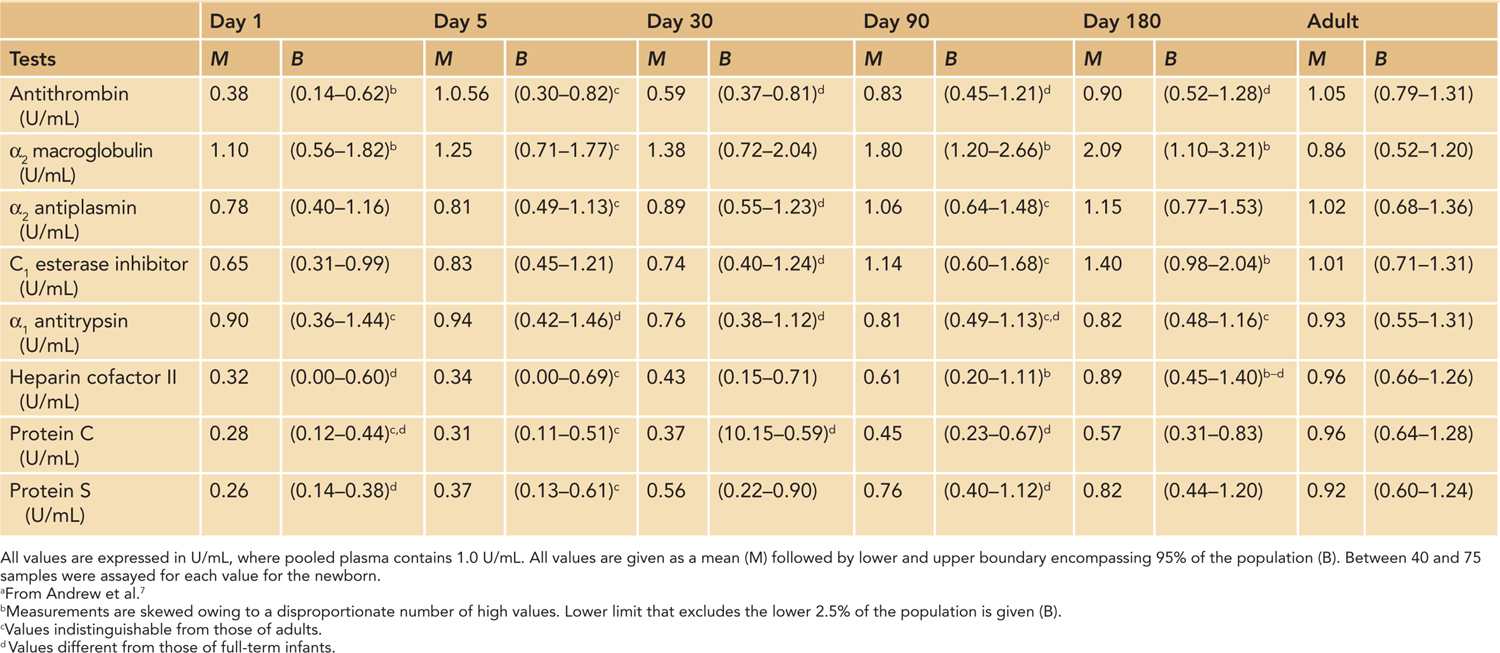
Prior to the 1980s, there was no concept of developmental hemostasis as it was presumed that children and adults had similar hemostatic systems. Dr. Maureen Andrew conducted 2 seminal studies to evaluate the human coagulation system in neonates. Her studies analyzed blood samples from 93 healthy, full-term infants and 137 healthy preterm infants born between 30 and 36 weeks estimated gestational age. At various time points throughout the first 6 months of life, she tested plasma concentration levels of coagulation and anticoagulation factors in the infants.
Her studies revealed that the neonatal hemostatic system evolves rapidly from birth through infancy. Plasma concentrations of coagulation and anticoagulation factors are drastically different from those found in adults. The results showed that levels of indigenous anticoagulants, such as antithrombin, protein C, and protein S, are less than 50% of the normal adult levels in the neonatal period, which is only somewhat balanced by an increase in serum concentrations of α2-macroglobulin (Tables 34-2 and 34-3). However, most of serum procoagulant concentrations are also decreased in newborns (see discussion in another chapter).
Table 34-2 Reference Values for the Inhibition of Coagulation in the Healthy Full-Term Infant During the First 6 Months of Lifea
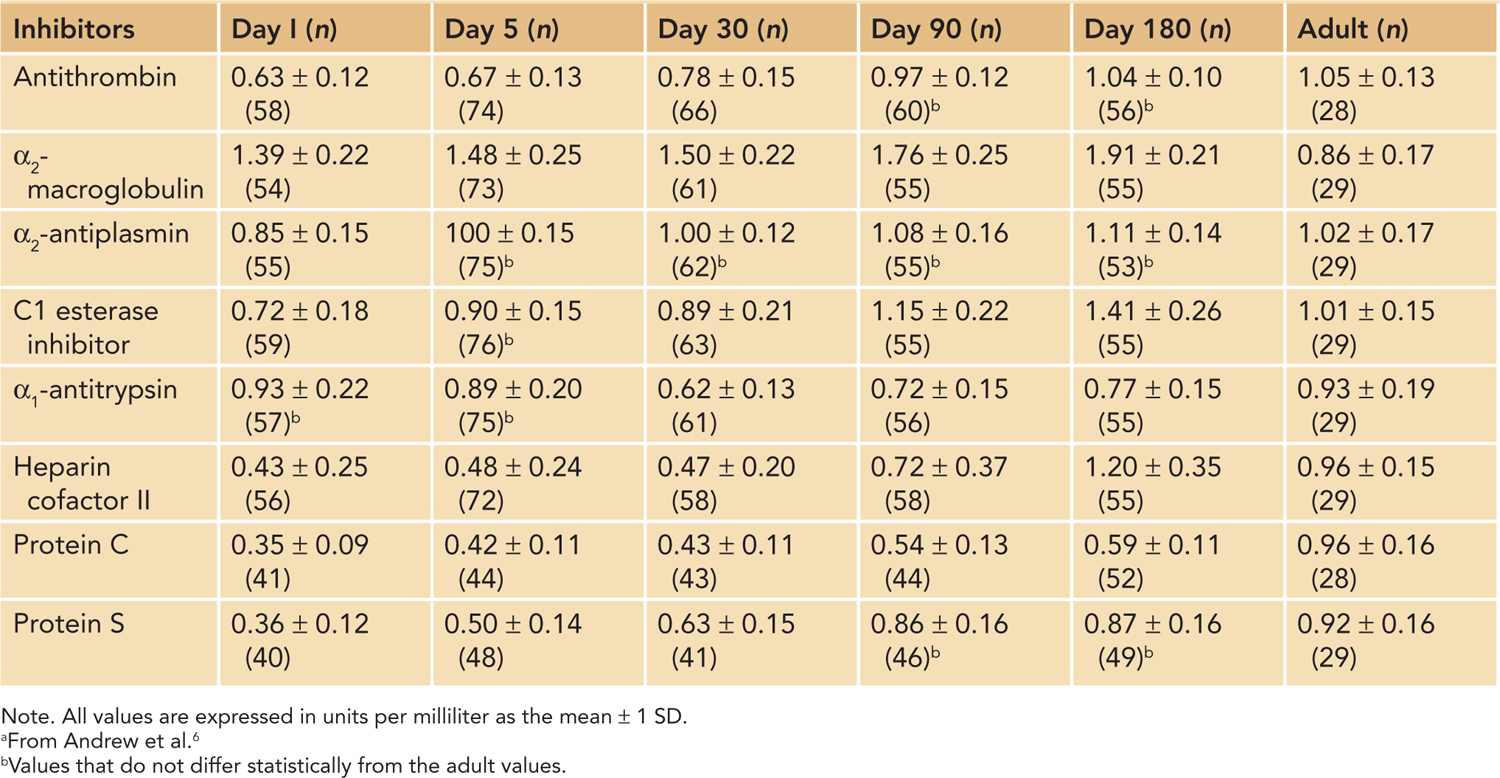
Table 34-3 Reference Values for Inhibitors of Coagulation in Healthy Premature Infants During First 6 Months of Lifea
In addition, the fibrinolytic pathway is altered in newborns. Although newborns have increased tissue plasminogen activator (tPA), plasminogen levels are about 50% of adult levels, and neonatal plasminogen has a qualitative impairment, requiring more tPA for activation. Also, plasminogen activator inhibitor (PAI) is increased, making the fibrinolytic pathway in infants less active.2
These alterations in pro- and anticoagulant concentrations form an equilibrium in healthy infants that prevents significant hemorrhagic or thrombotic complications. However, due to these variations, neonates, especially preterm ones, are at an increased risk of shifting this equilibrium toward bleeding or thrombotic complications in the presence of illness, instrumentation, or other hemostatic challenges.
EPIDEMIOLOGY
The exact incidence of neonatal thrombosis is unknown. A German registry of symptomatic neonatal thrombosis over a 2-year period revealed an incidence of 0.51 per 10,000 births.8 In a Canadian registry of cases submitted from neonatal intensive care units (NICUs) in North America, Europe, and Australia during a 3.5-year period, the incidence of clinically apparent thrombi, excluding stroke, in infants less than 1 month of age was 24 per 10,000 admissions.4 Approximately two-thirds to three-fourths of the events were venous thrombi. A 2-year registry of venous thromboembolism from the Netherlands reported an annual incidence of 14.5 per 10,000 children aged 0–28 days.5
Renal Vein Thrombosis
Renal vein thrombosis is the most prevalent non-catheter-related thrombosis in neonates; however, its exact incidence is unknown due to the lack of large population-based studies. The estimated incidence in Germany from 1992 to 1994 was 2.2 per 100,000 births, and it accounted for 22%–44% of thrombi reported in 2 large neonatal registries.4,8 Renal vein thrombosis is more prevalent in males, representing 67% of the cases. Although the cause of the gender difference is unknown, some hypothesize that it is due to differences in renal perfusion or the increased rate of congenital renal malformations in males.9
Renal vein thrombosis occurs in both preterm and full-term infants. The time of onset varies, with 67% of cases occurring within 3 days of birth. However, renal vein thrombosis presents antenatally in 7% of cases and in greater than 3 days after birth in 26% of patients.10 In 70% of cases, renal vein thrombosis is unilateral, and the majority of these cases (64%) involve the left kidney.10 These thrombi may extend into the inferior vena cava and are occasionally associated with adrenal hemorrhage. Although the classic triad of renal vein thrombosis includes macroscopic hematuria, palpable flank mass, and thrombocytopenia, all 3 are only seen in 13%–22% of cases.9
Perinatal risk factors have been found to be associated with renal vein thrombosis. Perinatal asphyxia has been associated in approximately 32% of cases.10 Renal vein thrombosis is also associated with dehydration, sepsis, congenital heart disease, and maternal diabetes mellitus. These conditions allow for thrombus formation through endothelial injury from hypoxia or endotoxin in the setting of decreased vascular blood flow. In addition, neonates with renal vein thrombosis are more likely to have an inherited thrombophilia.
Neonatal Purpura Fulminans
Neonatal purpura fulminans is a rare, life-threatening disorder that presents with dermal microvascular thrombosis associated with DIC. Often, these neonates present 2–12 hours after birth with cutaneous purpura, frequently initiating at the site of previous trauma. The lesions progress from dark red to indurated, purple-black. If left untreated, the areas will become necrotic and gangrenous. In addition to the dermal findings, the neonates may have intracerebral thrombi, vitreous hemorrhage, retinal detachment, and large-vessel thrombosis.
The symptoms are caused by a deficiency of protein C or S. This deficiency may stem from inherited risk factors, such as homozygous protein C or S deficiency, compound heterozygosity, or coinheritance with another inherited thrombophilia. Acquired causes are primarily attributed to infection, with group B Streptococcus the most common. Severe hepatic dysfunction, galactosemia, and severe congenital heart disease have also been associated with neonatal purpura fulminans.11
PATHOPHYSIOLOGY
The activation of the coagulation cascade leads to thrombus formation. Natural anticoagulants and the fibrinolytic system help to keep the hemostatic system in equilibrium. In neonates, several factors may interfere with the hemostatic system, leading to thrombosis. Some of these factors may be inherited, while others are acquired. Inflammation, leading to damage of the endothelial surface, and foreign bodies, such as catheters, can activate the coagulation cascade. In addition, changes in blood flow that lead to increased stasis may increase an infant’s propensity to clot. Often, multiple factors occur at the same time to tip the balance toward thrombosis. Since neonates have a more delicate hemostatic balance, they have a great propensity to develop thrombi with minimal disruptions.
Acquired Risk Factors
The majority of thrombi that develop in neonates occur due to acquired risk factors. Neonatal and maternal factors can lead to thrombosis (Table 34-1).
Catheter-Associated Thrombosis
The placement of central lines is almost ubiquitous in neonates admitted to the hospital. These lines are used for intravenous therapy, blood products, blood sampling and to measure arterial blood pressure. These catheters are typically placed in the umbilical vein, umbilical artery, or a peripheral vein. The thrombotic risks associated with these catheters are thought to be due to vessel injury, blocked blood flow due the large size of the catheter compared to the diameter of the vessel, and thrombogenic catheter materials.
Venous: Umbilical venous catheter (UVC) thrombosis has been documented in 22%–43% of neonates undergoing systemic ultrasound screening.1 Many times, they are asymptomatic; however, UVC thrombus should be considered in dysfunctional lines, lines with persistent infections, and in infants with persistent thrombocytopenia of unknown etiology. The position of line placement is crucial. UVCs should be inserted to the inferior vena caval–right atrial junction. Of right atrial thrombi, 91% occur in the setting of a central venous line.12 In addition, portal vein thrombosis is associated with a UVC approximately 75% of the time, and 50% of the time it is associated with aninappropriately placed UVC.13 Current recommendations are to replace a UVC after 14 days.
Arterial: Arterial thrombi in the neonate are predominantly iatrogenic due to the presence of catheterization of the umbilical, femoral, and peripheral arteries. Studies using ultrasound and angiography evaluation reported an incidence of 14%–35% and 64%, respectively.2 The majority of these thrombi occur several days after line removal. High-positioned catheters and end-hole catheters tend to have fewer clinical vascular complications. Spontaneous arterial thrombi occur in the aorta of the neonate occasionally, but this is exceedingly rare.
Other Infant Factors
Conditions that cause a hemostatic imbalance place neonates at risk for thrombosis due to alteration in blood flow. Congenital heart disease, asphyxia, dehydration, and polycythemia are all known risk factors. In addition, infection and sepsis increase the risk of thrombus formation by activating clotting factors through inflammation and DIC. Long-term administration of total parenteral nutrition (TPN) can increase endothelial damage and lead to thrombus formation at the catheter tip. Other events that damage the endothelium, such as surgery, can lead to increased risk in the neonate. Infants on extracorporeal membrane oxygenation (ECMO) are at increased risk of thrombosis due to alteration of blood flow as well as endothelial damage. Many of these factors occur in combination with the placement of an intravascular catheter, further increasing the risk to the neonate.
Antiphospholipid Syndrome (APS)
The mother and fetus share their circulatory systems through the placenta. There is an association between thrombosis and infarction of the placenta and thrombosis and infarction in the neonate that is thought to be due to embolization. APS, which is known to cause thrombosis in children and adults, is a rare cause of thrombosis in neonates and occurs in neonates of affected women. APS is defined as the presence of an antiphospholipid antibody and a disease manifestation, including thrombosis, recurrent miscarriages, or premature birth due to preeclampsia, eclampsia, or placental insufficiency. APS can be a primary disorder or associated with autoimmune disorders, such as systemic lupus erythematosus. The antibodies associated with APS include anticardiolipin antibody, lupus anticoagulant, and β2-glycoprotein-I antibody. APS in the mother may be diagnosed after the thrombotic complication in the neonate is observed.
The majority of neonatal thrombi associated with APS are arterial, and many involve the central nervous system. Anticardiolipin antibody, lupus anticoagulant, and β2-glycoprotein-I antibody have all been associated with APS in infants. These antibodies are usually tested in the mother, though they can be detected in the affected infant due to transplacental transfer of the immunoglobulin (Ig) G antibodies. Infants with APS often have additional neonatal risk factors for thrombosis, including catheters, asphyxia, or sepsis.14
Maternal Factors
Many maternal factors other than APS may lead to increased risk of neonatal thrombosis. Preeclampsia and gestational diabetes can lead to endothelial damage, which may predispose the placenta to thrombosis. Similarly, chorioamionitis is associated with inflamed placental vessels and localized thrombosis. Maternal smoking and polydrug use can lead to a variety of placental changes, including vasospasm, endothelial activation, and thrombocytosis. Vascular changes to the placenta may disrupt the delicate hemostatic balance in the infant or lead to thrombosis due to placental embolism.2
Inherited Thrombophilias
Inherited thrombophilias are a well-known cause of thrombosis in children and adults. The role that they play in the formation of thrombi in neonates is not well defined. These conditions include protein deficiencies, such as antithrombin, protein C, and protein S. In addition, genetic mutations, including factor V Leiden, prothrombin gene mutation, and methylenetetrahydrofolate reductase (MTHFR) polymorphisms, play a role in thrombosis.
Antithrombin Deficiency
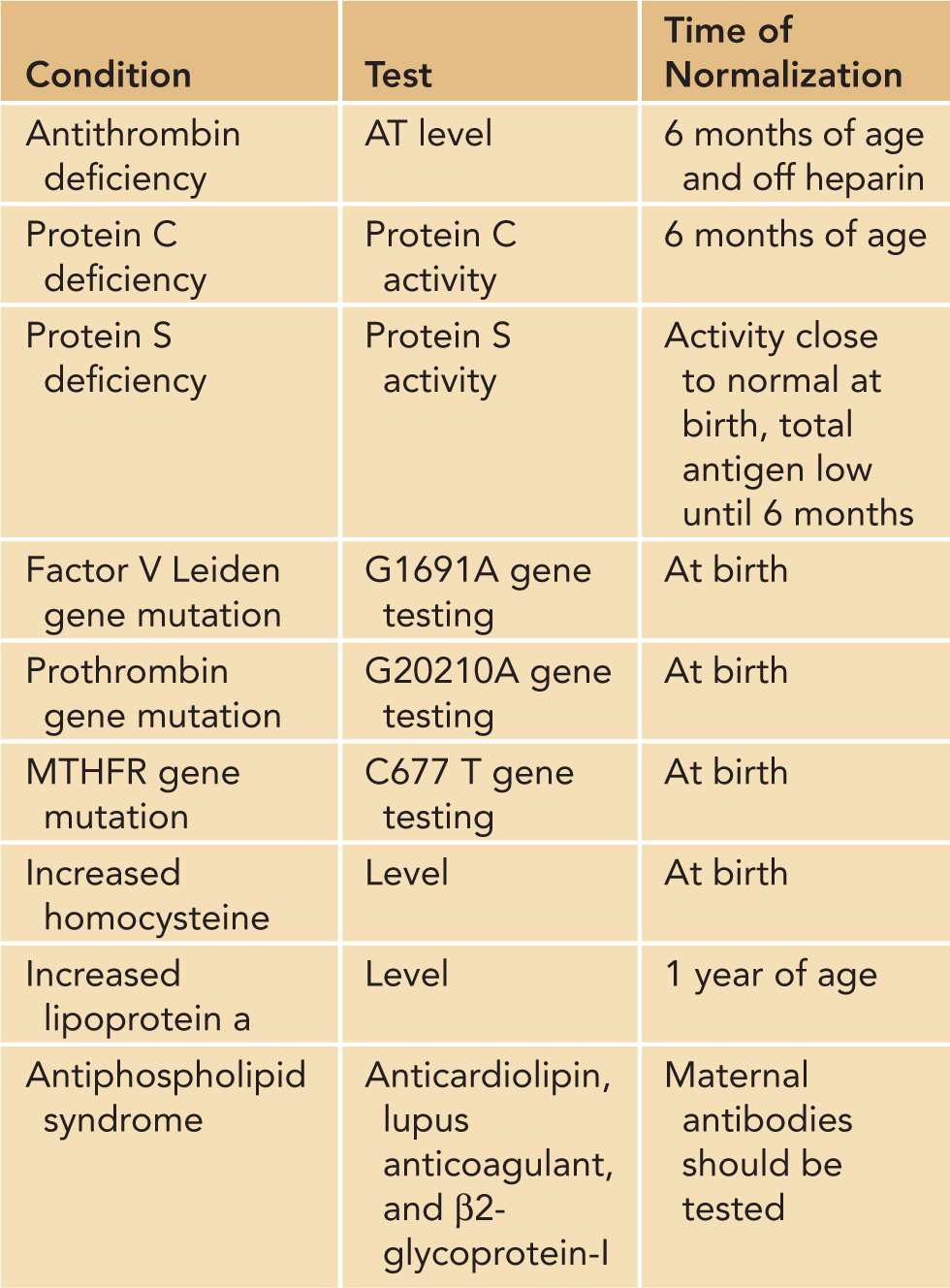
Antithrombin, formerly known as antithrombin III, is a vitamin K–independent protein primarily synthesized in the liver, which inhibits thrombin, factor Xa, and other coagulation serine proteases. A deficiency in this protein leads to a dysregulation of the serine proteases involved in the coagulation cascade, leading to a prothrombotic state. The gene encoding antithrombin has been localized to chromosome 1. Mutations in this gene may lead to either a quantitative (type I) or qualitative (type II) deficiency. Familial antithrombin deficiency is inherited in an autosomal dominant fashion, with the homozygous state almost universally fatal in utero. Heterozygous deficiencies occur in 0.02%–0.2% of the general population.15 In a recent meta-analysis of pediatric patients with deep vein thrombosis, there was an odds ratio of 9.4 for antithrombin deficiency.16 It is important to note that neonates have a physiologically lower level of antithrombin, which may affect screening for the deficiency (Table 34-4).
Table 34-4 Thrombophilia Testing and Time Until Normalization
Protein C Deficiency
Protein C is a vitamin K–dependent protein synthesized in the liver. It forms an activated complex in conjunction with a protein S cofactor called activated protein C (APC), which prevents excess coagulation through the inactivation of factors Va and VIIIa. Protein C deficiency is due to a mutation on chromosome 2, which may be a quantitative (type I) or qualitative (type II) deficiency. Asymptomatic protein C deficiency occurs in 0.2%–0.5% of the population; clinically significant mutations occur in 1/20,000 individuals.17 The deficiency is inherited in an autosomal dominant fashion; however, severe forms, including homozygotes and complex heterozygotes, are less frequent than predicted, presumably due to fetal demise. Protein C levels may be difficult to interpret in the neonate due to physiologically low levels (Table 34-4).
Protein S Deficiency
Protein S is an important vitamin K–dependent cofactor in the protein C complex that is also synthesized primarily in the liver. Deficiencies in this protein lead to a hypercoagulable state by decreasing the function of APC. Protein S deficiency is due to a mutation on chromosome 3 that is inherited in an autosomal dominant fashion. The quantitative type I and III deficiencies are much more common than the rare qualitative type II deficiency. Heterozygous protein S deficiency occurs in 1%–13% of all individuals with venous thrombosis.18 Homozygous individuals are extremely rare as homozygosity is usually incompatible with life. Total protein S levels are markedly decreased in newborns, though functional levels may only be slightly reduced. Care should be taken in interpreting these values in newborns (Table 34-4).
Factor V Leiden Mutation
Factor Va Leiden, named for the town in which it was first discovered, is the most common cause of inherited thrombophilia. Factor Va Leiden mutation confers resistance of factor Va to inactivation by APC, leading to a hypercoagulable state. It is an autosomal dominant point mutation in the factor V gene on chromosome 1 that is characterized by a guanine-to-adenine substitution at position 1691 (G1691A). This mutation occurs in 0.5%–5% of the population, with Caucasians having the highest frequency. Homozygosity occurs in 1 in 5000 individuals and leads to a higher incidence of thrombosis at a younger age.19
Prothrombin G20210A Mutation
Prothrombin gene mutation, which leads to the substitution of an adenine for guanine at the 20210 position of the prothrombin (factor II) gene on chromosome 11, leads to increased levels of prothrombin. This increase in plasma prothrombin levels confers an increased thrombotic risk. Prothrombin G20210A mutation is autosomal dominant and occurs in approximately 2% of the US population. Like factor V Leiden, it is more prevalent in Caucasians. Approximately 1 in 10,000 individuals is a homozygote, which confers a higher rate of thrombosis.19
Homocysteine
Increased homocysteine levels have been associated with both venous and arterial thrombosis. A common genetic polymorphism that can affect homocysteine levels is mutation in the MTHFR gene. MTHFR is an enzyme that converts 5,10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate. 5-Methylenetetrahydrofolate is a cofactor that is utilized in the conversion of homocysteine to methionine. A common polymorphism in the gene encoding for MTHFR, in which a cytosine is replaced with a thymine at position 677 (MTHFR C677T), is found in many populations. This polymorphism confers a decrease in activity of the MTHFR and thus lower availability of 5-methylenetetrahdrofolate and an increase in homocysteine levels. Therefore, testing for either MTHFR C677T mutations or, more directly, elevated homocysteine levels may be indicated in neonates with thrombosis. Both the heterozygous and homozygous states are commonly encountered, with the latter conferring a higher thrombophilic risk compared to the heterozygote state. However, MTHFR mutation does not confer risk without elevation in homocysteine.19
DIAGNOSTIC TESTING
Radiologic Testing
Thrombi are diagnosed using imaging. The gold standard for diagnosis of a thrombus is angiography; however, angiography requires contrast and radiation. Ultrasounds and echocardiograms are often used instead of angiography as they can be performed at bedside in the case of critically ill neonates. In studies, both ultrasound and echocardiography have been shown to have lower sensitivity when compared to venography.1 Similarly, the incidence of arterial thrombosis was found to be 14%–35% using ultrasound and 64% with angiography.2 This decrease in sensitivity may be attributed to the difficulty in interpreting whether the reduced compressibility of the vessel by the ultrasound probe is due to the catheter or a thrombus. In addition, the low pulse pressure of ill neonates may limit interpretation.
Renal Vein Thrombosis
Ultrasonography has replaced venography for the diagnosis of renal vein thrombosis as the sensitivity is high in evaluating the renal veins. The ultrasound findings vary depending on the time of onset and severity. Echogenic streaks are seen within the first few days and correlate to intralobular and interlobular thrombosis. In addition, the corticomedullary junction may be lost. Early changes, which typically occur in the first week, include renal enlargement with increased echogenicity of the renal parenchyma. During the second week, intermediate changes including prominent and diffuse renal enlargement and loss of corticomedullary differentiation are seen. At this time, there may be patchy hyperechoic and hypoechoic areas that represent hemorrhage and edema. After the first 2 weeks, the kidney may become normal in appearance or begin to atrophy. Thrombi may become calcified. Doppler images can detect high arterial resistance and reversal of flow. Currently, studies are trying to determine if ultrasound can be used to predict long-term prognosis in renal vein thrombosis.9
Thrombophilia Screening
The role of inherited thrombophilia in neonatal thrombosis is unclear. While some studies have found an increased association of inherited thrombophilia with catheter-related thrombosis, others have not.2 Several inherited thrombophilias have been found in association with neonatal renal vein thrombosis.9 In addition, neonatal purpura fulminans is often associated with protein C or S deficiency. Therefore, thrombophilia screening should be reserved for neonates with significant spontaneous thrombosis, recurrent thrombosis, or neonatal purpura fulminans.
Prior to undergoing thrombophilia screening, a detailed family history should be obtained, including the pregnancy history of the mother focused on miscarriages and premature births. Neonates with spontaneous thrombosis, recurrent thrombosis, or a strong family history of thrombosis should undergo the testing outline in Table 34-4. However, it must be noted that the levels of natural anticoagulants are decreased both during infancy and in the presence of a thrombus. Antithrombin, protein C, and protein S levels should be postponed to 6 months of age or repeated at that time if initial levels are low. In addition, lipoprotein a levels do not reach adult levels until approximately 1 year. If the level is low or normal, it should be repeated at 1 year of age.
In patients with neonatal purpura fulminans, protein C and S activity levels should be sent prior to treatment. If an individual is a homozygote, the level will be undetectable. As repeat testing in 3 to 6 months is not practical in these patients, testing the parents for heterozygous states is imperative and may require repeat analysis.
MANAGEMENT
The majority of neonatal thrombi are managed medically. Prior to initiating therapy, it is important to weigh the risks and benefits of antithrombotic therapy. Occasionally, surgical intervention may be considered in arterial and atrial thrombi; however, this is technically difficult due to the size of neonatal vessels. The choice of antithrombotic treatment is based on the location, size, and symptoms associated with the thrombus.
Medications
Currently, there are 2 common agents used for anticoagulation in neonates: unfractionated heparin and low molecular weight heparin, such as enoxaparin. Vitamin K antagonists, such as warfarin, are rarely used in infants due to the narrow therapeutic index and multiple dietary and medication interactions. In addition, there is concern for inconsistent dosing from crushing tablets as warfarin cannot be safely compounded. Newer anticoagulation agents, such as direct thrombin inhibitors and fondaparinux, are currently in development. The direct thrombin inhibitors, including bivalirudin and argatroban, have the advantage of being antithrombin independent; however, their study in neonates has been limited, and they require continuous administration. Fondaparinux is a synthetic antithrombin-dependent inhibitor of factor Xa that has a prolonged half-life, allowing for daily administration; however, dosing in neonates has not been established. In settings of life- or limb-threatening thrombosis, anticoagulation may not be enough. In these cases, thrombolytic agents, such as tPA, may be warranted if surgical thrombectomy is not possible.
Unfractionated Heparin
Unfractionated heparin works as an anticoagulant by potentiating the inhibitory effects of antithrombin on thrombin and factor Xa. Heparin is the treatment of choice for anticoagulation in the critically ill and is also used prophylactically to maintain catheter patency. It has a short half-life of 30 minutes and therefore must be administered continuously.
Due to high interpatient variability in dosing, it is essential to monitor for therapeutic activity levels. A heparin activity level, which is antifactor Xa, should be checked 6 and 12 hours after initiation of therapy and 6 hours after any adjustment to the dose. The heparin infusion should be adjusted to obtain a goal heparin activity level of 0.35–0.7. Once the level is therapeutic, it should continue to be monitored twice daily. Activated partial thromboplastin time (PTT) is not suggested for monitoring as levels may vary based on the age of the neonate and are not as reliable for evaluating the effects of heparin. Part of the variability in heparin activity is because infants are physiologically deficient in antithrombin. Antithrombin can be safely replaced to allow for better anticoagulation effects of heparin. Levels should be monitored to ensure they remain adequate (greater than 60%).
Heparin therapy is not without side effects. The main adverse effects associated with heparin therapy are associated with bleeding. Advantages of unfractionated heparin are that the half-life is short and the infusion can be stopped. In addition, protamine sulfate serves as a reversal agent by binding heparin. However, another major adverse effect is heparin-induced thrombocytopenia (HIT). HIT is initiated by the formation of antibodies to heparin that activates platelets, leading to further thrombus formation and thrombocytopenia. The incidence of HIT in neonates, particularly those with congenital heart disease, is approximately 1% and can lead to significant morbidity and mortality.20
Enoxaparin
Enoxaparin, or Lovenox®, is the most commonly used low molecular weight heparin. Like unfractionated heparin, enoxaparin works by potentiating the effects of antithrombin. However, it has a more profound effect on factor Xa than on thrombin. Enoxaparin is becoming more popular in the treatment of neonatal thrombosis due to the ease of dosing. It is administered via a subcutaneous injection and has a longer half-life than heparin, allowing twice-daily dosing. However, unlike heparin, enoxaparin does not have a reliable mechanism of reversal with protamine; therefore, it should not be used in infants requiring immediate invasive interventions or surgical procedures. In the case of a planned procedure, enoxaparin should be discontinued 24 hours prior to the event. In addition, enoxaparin is not recommended in premature infants who do not have sufficient subcutaneous tissue for administration.
In general, preterm neonates require higher doses of enoxaparin compared to term infants to reach the therapeutic goals. Similarly, infants require increased doses compared to older children and adults. These changes in pharmacodynamics may be due to the larger volume of distribution in infants and the developing hemostatic system. In addition, rapid weight gain in neonates contributes to the difficulty in maintaining therapeutic dosing once it is achieved. The current recommended starting dose of enoxaparin in neonates is 1.5 mg/kg every 12 hours; however, prospective and retrospective studies have suggested that doses of 1.7 mg/kg every 12 hours in term neonates and 2 mg/kg every 12 hours in preterm neonates allow for more rapid attainment of therapeutic levels without an increased number of bleeding events.21 The increased dosage would not be suggested in infants with high risk of bleeding.
Enoxaparin requires antifactor Xa monitoring to ensure that levels remain in the therapeutic range of 0.5–1 U/mL. Levels should be checked 4 hours after the dose. Like heparin, enoxaparin works by activating antithrombin; therefore, it is important to ensure that the antithrombin level is adequate (greater than 60%) during therapy. While enoxaparin monitoring tends to require fewer venipunctures than heparin, obtaining therapeutic targets with enoxaparin poses a similar challenge to heparin therapy. Once levels are therapeutic, antifactor Xa monitoring should be performed at a minimum of weekly to ensure proper dosing.
Adverse events documented with the use of enoxaparin in infants primarily are bleeding events. Minor events, including bruising and oozing from the injection site, occurred in 0%–56% of patient cohorts. Major bleeding events occurred in 4% of neonates and included major bleeding at the injection site, compartment syndrome, bleeding from gastrointestinal ulcers, intracerebral hemorrhage, hemorrhagic infarction, and hemorrhagic pericardial effusion. All of these occurred while the enoxaparin level was therapeutic.21 Enoxaparin can also affect bone metabolism, leading to osteopenia after prolonged periods of use, although there are no studies of this in infants. In addition, HIT can develop with the use of enoxaparin, though it occurs less with low molecular weight heparin compared with unfractionated heparin.
Thrombolytic Agents
Although anticoagulation is the mainstay of medical treatment in neonates with thrombosis, thrombolytic agents may be indicated in cases of thrombi obstructing blood flow to a limb or organ. These agents should be considered in thrombi causing tissue ischemia, superior vena cava syndrome due to thrombosis, massive pulmonary embolism, bilateral renal vein thrombosis, large atrial thrombi, congenital heart disease with shunt obstruction, and cerebral sinovenous thrombosis with progressive decline.22 Thrombolytic drugs work by converting plasminogen to plasmin in order to promote fibrinolysis. They are most effective in thrombi that have been present for less than 2 weeks. tPA is the most common thrombolytic used in children and neonates; however, few clinical trials used these agents in neonates.
Thrombolytic agents can be used either systemically or locally. There are 2 distinct systemic dosing regimens that are commonly used for tPA. High-dose tPA consists of a 0.5–0.6 mg/kg/h infusion for 6 hours followed by an evaluation to determine if the course should be extended or repeated. This is commonly used for life- or limb-sparing thrombi. More recently, there is evidence that low-dose tPA may be as effective in treating peripheral venous and arterial thrombi. Low-dose tPA typically starts at 0.03 mg/kg/h with an hourly maximum of 2 mg and may be increased to 0.06 mg/kg/h. This infusion may be continued for 48 to 96 hours.22 Catheter-directed thrombolysis is an alternative with decreased systemic effects by allowing delivery of the medication directly to the affected site. However, placement of a catheter in the affected area may be difficult in small neonates. There is no standard dosing range for catheter-directed therapy, though tPA doses of 0.015 to 0.2 mg/kg/h have been reported.23
The utility of thrombolytic therapy has been extrapolated in review articles that included multiple different types of thrombi and courses of treatment. In a review of 413 children of all ages who received thrombolytics, 64% of patients had complete clot resolution, 15% had partial resolution, and 21% had no resolution.22 The most concerning factor limiting the use of these agents is the increased risk of bleeding, especially in neonates who are at a higher risk for intracranial hemorrhage. In the same cohort, 22% of the patients had a minor bleed, and 15% had a major bleed, defined as central nervous system hemorrhage or other hemorrhage requiring blood transfusion. Fatal bleeding occurred in 1.25% of these patients.22 In a separate review of cases reported in the literature, intracranial hemorrhage was found in 14/929 children (1.5%) but was more common in preterm infants, with a prevalence of 25%.22 To decrease the risk associated with thrombolytic therapy, a number of relative contraindications have been formulated (Table 34-5). The risk of bleeding due to one of these factors should be weighed against the risk of the thrombus.
Table 34-5 Contraindications for Use of Thrombolytic Therapya



