Characteristic
Type I
Type II
Chronic estrogenic stimulation
Present (hormone dependent)
Absent (not hormone dependent)
Growth
Slow growing
Rapid
Precursor lesion
Atypical hyperplasia
Endometrial intraepithelial carcinoma
Age at initial diagnosis
Pre-/perimenopausal
Postmenopausal
Built
Obese
Thin built
Histology
Endometrioid
Serous, clear cell
Grade
Low
High
Depth of invasion
Usually superficial
Often deep
ER, PR
>90 %
0–31 %
5-year survival rates
FIGO stage I/II/
III/IV
85–90 %/70 %/
40–50 %/15–20 %
60 %/50 %/
20 %/5–10 %
Molecular aberration | Type I | Type II | Aberrant pathway |
|---|---|---|---|
PTEN (loss of function through deletion or mutation) | 50–80 % | 10–11 % | PI3K/AKT/mTOR pathway |
P53 mutations | 5–10 % | 80–90 % | Tumor suppressor gene |
HER-2/neu (overexpression) | 3–10 % | 32–43 % | Cell surface receptor |
P16 inactivation | 10 % | 40 % | Cyclin D/CDK4-CDK6/RB |
EGFR expression | 46 % | 34 % | Cell surface receptor |
Ploidy | 67 % diploid | 45 % diploid | Aneuploid associated with poor prognosis |
55 % aneuploid | |||
K-ras (mutational activation) | 13–26 % | 0–10 % | Ras-Raf-Mek-Erk pathway |
E-cadherin (loss or non expression) | 10–20 % | 62–87 % | Wnt/-catenin./LEF-1 pathway |
Catenin CTNNB1 (gain of function mutation) | 25–38 % | Rare | Wnt/-catenin./LEF-1 pathway |
HIF1a overexpression | 25 % | 80 % | Gene transcription nuclear protein |
EpCAM overexpression | Unknown | 96 % | Transmembrane protein |
Standard Treatment
Standard treatment almost universally begins with a total hysterectomy (via any of a number of approaches—abdominal, vaginal, or minimally invasive) and removal of the remaining adnexal structures. Comprehensive staging, including pelvic and para-aortic lymph node assessment, is crucial in guiding postoperative adjuvant treatment (Table 29.3). EC spreads beyond the uterus by infiltrating directly through the myometrium, extending into the cervix or metastasizing, most often to the pelvic nodes and less frequently to the para-aortic nodes [3, 4].
Stage | Recommendation |
|---|---|
Stage IA, grade I–II | Total abdominal hysterectomy, bilateral salpingo-oophorectomy, lymph node sampling |
Stage IA, grade III, IB, stage II | Total abdominal hysterectomy, bilateral salpingo-oophorectomy followed by pelvic radiation |
Stage III A, Gr I–II | Total abdominal hysterectomy, bilateral salpingo-oophorectomy followed by pelvic radiation |
Stage III A, high grade, III B,C | Total abdominal hysterectomy, bilateral salpingo-oophorectomy followed by chemotherapy and pelvic radiation |
Stage IV | Systemic therapy: chemotherapy or progestins |
Adjuvant postoperative treatment recommendations in advanced stage disease are widely disparate and an area of active research. However, assuming an adequate performance status, virtually all women with advanced stage disease (stage III and IV) will be recommended for chemotherapy, external beam pelvic radiotherapy with or without an extended para-aortic field, or some combination of both modalities. These treatments are geared at improving disease-free and overall survival in a population in whom overall survival remains disappointing—as low as 20 % in stage IV disease [7]. The role of surgical lymphadenectomy has been continuously debated since the FIGO staging criteria were adopted. Specifically, identifying which patients benefit from lymphadenectomy represents a unique challenge [4].
Type II EC-serous and clear cell carcinoma is biologically similar to high-grade serous carcinoma of ovary with high propensity for upper abdomen relapse. Currently, there is lack of prospective, randomized trials for type II EC. From available literature, there is suggestion that surgical staging should be performed even in the setting of minimally invasive/noninvasive disease. For all patients of type II EC-serous papillary or clear cell histology, chemotherapy should be included in both early stage and advanced disease. The role of radiation in combination is unclear and is currently being investigated in GOG 249 and GOG 258 protocols [8]. Currently, paclitaxel- and carboplatin-based protocol (similar to epithelial ovarian cancer) is being used [4–6].
Treatment of Recurrent and Metastatic Disease
Local Recurrence
Disease usually recurs within the first 3 years following initial treatment. After the diagnosis of the recurrence, a complete evaluation (including imaging) for assessment of the disease extent is important. Surgery is considered only in solitary/isolated recurrences (e.g., single lung metastasis) and in cases where it is hoped it will improve the patient’s symptoms and quality of life.
Pelvic recurrences are most commonly found at the vaginal vault. Selected patients with vaginal recurrence who have not received radiation earlier can be treated with radiation with complete response rate of 40–81 %. If the remaining tumor after pelvic RT is <3–5 mm, intracavitary brachytherapy can be used. Otherwise, interstitial brachytherapy can be considered if available. Small central pelvic recurrence within a radiation field can be treated with pelvic exenteration [4–6] (Fig. 29.1).
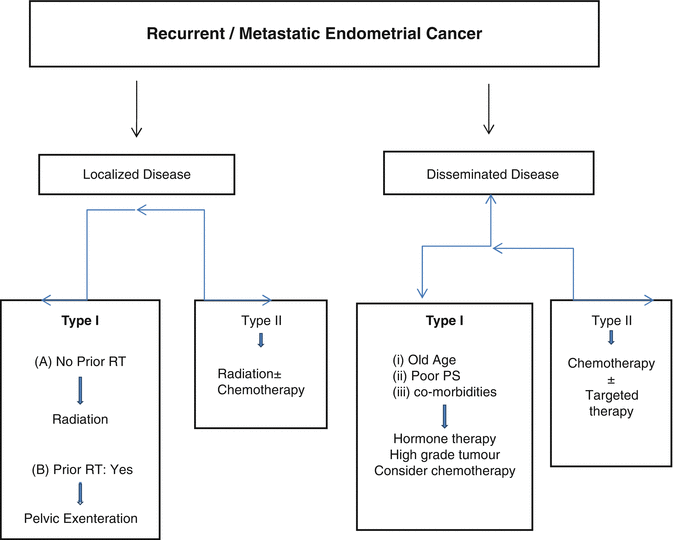

Fig. 29.1
Algorithm for the management of recurrent/metastatic Endometrial cancer
Metastatic or Disseminated Disease
Patients with low-grade disease with estrogen receptor (ER) and progesterone receptor (PR) positive EC tend to respond to hormonal therapy as well as chemotherapy. Hormonal therapy may be preferred in patients with poor performance status and/or medical comorbidities. Cytotoxic chemotherapy is preferred in patients with high-grade EC [3–5].
Hormone Therapy
Hormone therapy is effective in type I endometrial cancer (those with endometrioid histology); ER/PR status in metastatic disease predicts response to hormone therapy. Progestational agents that have been used in treatment of recurrent/metastatic disease include hydroxyl-progesterone, medroxyprogesterone, and megestrol. These agents produce a partial or complete response rate of 20–29 %. Long-term exposure to progestins leads to downregulation of ligand-dependent activation of PR and may lead to loss of response within the endometrium. In a Gynecology Oncology Group (GOG) study, 61 patients with advanced or recurrent uterine cancer were treated with a combination of megestrol and tamoxifen (antiestrogen). This strategy was based on hypothesis that intermittent exposure to progestins would permit tamoxifen to induce progesterone receptor and thus enhance effect of progestin therapy. The complete response rate was 21 %, and 5.4 % had a partial response. The average survival was 14 months. Toxicity was moderate and there were no treatment-related deaths. Of those who responded, 50 % sustained this response for an average of 20 months. It was noted that, overall, younger women had better responses to the treatment than older women [4, 5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


