Route of recurrence
Risk factors
Hematogenous
All stages of disease
Myometrial invasion >50 %
Stage I disease, negative LNs
Myometrial invasion ≥66 %
Lymphatic
Pelvic/paraaortic LNs
CSI, LN metastases
Peritoneal spread
Stage IV disease
Stages Il-III disease, ≥2 CSI, PPC, LN metastases, or type II histology
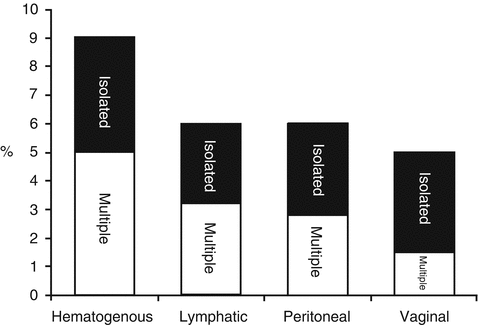
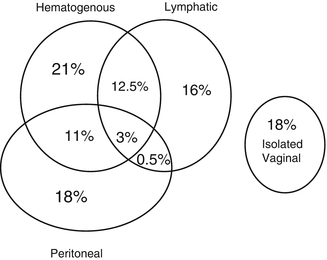
Fig. 18.1
Distribution of sites of recurrence in 915 endometrial cancer patients with 21 % relapse rate (Adapted from Mariani et al. [18]; used with permission)
It is notable that in patients deemed to be at risk for lymph node metastases who undergo staging, the overall rate of lymph node metastasis is 22 % (84 % and 67 % have pelvic or paraaortic lymph node metastases, respectively) [14]. Furthermore, patients diagnosed with retroperitoneal lymph node metastases or cervical invasion have a significantly higher incidence of lymph node involvement and a poorer prognosis than patients with disease confined to the uterus [3, 23, 24]. In a study of 112 patients with positive pelvic and or paraaortic lymph nodes, the external iliac and obturator lymph node basins were the most commonly involved pelvic lymph node sites in patients with disease confined to the corpus, while patients with cervical invasion were significantly more likely to have positive common iliac lymph nodes [23]. In fact, all patients with both cervical involvement and positive paraaortic lymph nodes were noted to have involvement of the common iliac nodes [23]. Thus, the presence of positive paraaortic lymph nodes correlates highly with common iliac node involvement [25]. Moreover, paraaortic lymph nodes are in direct communication with the external iliac and obturator basins, and drainage into the systemic circulation through the paraaortic lymph nodes is central to disease spread [25–27]. Metastases from the uterine corpus may be associated with direct extension to the paraaortic lymph node basin via gonadal vessels and the infundibulopelvic ligament [23].
Pelvic lymph node status is one of the strongest surrogate markers of paraaortic lymph node involvement [19, 25, 28]. When pelvic lymph nodes are negative, only 2 % of patients are found to have positive paraaortic nodes [19, 29]. In addition, LVSI, which is present in 50–90 % of patients with positive paraaortic lymph nodes, in combination with positive pelvic lymph nodes was found to be highly predictive of paraaortic lymph node metastases [29]. The overall prevalence of positive paraaortic lymph nodes has been described to be between 5 % and 15 % in early-stage disease, and paraaortic lymph nodes may be involved in the absence of pelvic lymph node metastases in 1–6 % of patients [8, 25, 28]. Patients with grade 2 or 3 endometrioid EC, ≥50 % myometrial involvement with or without macroscopic extrauterine disease are most at risk for isolated positive paraaortic lymph nodes [15]. Microscopic paraaortic disease and the presence of isolated tumor cells have additionally been described in up to 73 % of patients with positive pelvic lymph nodes [30, 31].
High tumor grade and deep myometrial invasion have been identified as key factors associated with lymph node metastases [8], while patients with grade 1 or 2 endometrioid tumors and/or superficial myometrial invasion (<50 %) are deemed to be at low risk for lymph node invasion [8, 32]. A rate of lymph node invasion of 4–5 % has been historically described in this group of patients and is considered by most gynecologist to be high enough to warrant lymphadenectomy [33]. However, this rate does not take into consideration tumor diameter, which may potentially lead to an overestimation of risk. Prior observations have shown that patients with primary tumor diameter >2 cm had a 7–8 % risk of regional lymph node involvement in comparison to 0 % in patients with tumor diameter <2 cm [34]. It is recommended that intraoperative tumor diameter be determined through notation of the size of the primary lesion in the three largest dimensions, where primary tumor diameter is defined as the largest of the three dimensions of the tumor. In cases where more than one lesion is present, the lesion with the largest diameter is considered [14, 35, 36]. Furthermore, when the endometrium is diffusely involved by tumor or is abnormal in gross appearance, the size of the tumor is approximated with the aid of microscopic examination of tumor sections [35].
Adequacy of Lymphadenectomy
It has been reported that extensive lymph node resection is associated with improvement in disease-specific survival. In a large cohort of patients with stage I–IV EC with intermediate- and high-risk factors (stage IB, grade 3, or stage IC, II–IV all grades), a 5-year survival benefit of over 30 % was accrued when more than 20 lymph nodes were resected compared to 2–5 lymph nodes [37]. Furthermore, some authors have shown that removal of more than 10 lymph nodes in both low-risk and high-risk patients has diagnostic and prognostic benefits [38, 39]. In a Japanese study of stage IIIC EC patients, patients who had two or more positive pelvic lymph nodes resected had a significantly improved disease-specific survival at 5 years if paraaortic lymphadenectomy was performed compared to pelvic lymphadenectomy alone [40, 41]. This supports the notion that lymphadenectomy may be therapeutic if thoroughly performed. It has been demonstrated that resection of 21–25 pelvic lymph nodes provided an 80 % probability of detecting at least one positive lymph node [42, 43].
Evidence to support performance of comprehensive pelvic and paraaortic lymphadenectomy is limited to patients with intermediate- or high-risk features (FIGO grade 3, deep myometrial invasion, lymphovascular space invasion, or macroscopic extrauterine disease). A significant survival advantage was demonstrated by US and Japanese groups for high-risk patients who underwent paraaortic lymphadenectomy in addition to pelvic lymphadenectomy [18, 44, 45]. In comparison, the initial PORTEC trial demonstrated that patients with stage I disease and high-risk features (deep myometrial invasion and grade 3) who were treated with hysterectomy and adjuvant pelvic external beam radiation alone had a 31 % risk of distant recurrence [46, 47]. Proponents of comprehensive surgical staging for all EC patients believe that full staging precludes the need for postoperative radiotherapy [48, 49]. However, there is currently no precedence for the use of adjuvant external beam radiation therapy in low-risk patients [50]. Thus, the literature suggests that only patients with substantial clinical and pathologic risk factors are likely to gain benefit from comprehensive surgical staging [45].
The anatomic extent and defining landmarks of paraaortic lymphadenectomy have not been clearly defined among the gynecologic oncology community. According to an SGO survey, 50 % of gynecologic oncologists use the IMA as the upper extent of paraaortic lymph node dissection, and only 11 % perform paraaortic dissection to the level of the renal vessels [13]. It has been shown that in patients with positive paraaortic nodes, positive nodes located above the inferior mesenteric artery (IMA) are prevalent in 73 % of patients [25]. In addition, 77 % of patients with positive paraaortic lymph nodes harbor metastases above the IMA and 63 % of patients with positive lymph nodes below the IMA also have positive nodes above the IMA. Limiting dissection to the IMA may potentially miss up to 46 % of patients with positive paraaortic nodes [15]. We therefore suggest that systematic pelvic and paraaortic lymphadenectomy should include dissection to the renal vessels with at least 22 pelvic lymph nodes and 10 paraaortic lymph nodes removed. These guidelines apply to high- and intermediate-risk patients.
Quality Assessment and Risk Stratification in Endometrial Cancer
Continuous evaluation of the quality of surgical care and the reliance on defined surgical guidelines allows for improved surgical staging [42]. It has been demonstrated that implementation of surgical guidelines and continuous quality assessments results in an improvement in compliance with pelvic and paraaortic lymphadenectomy. The identification of a minimal number of lymph nodes removed has been suggested to be an essential component of clinical trials involving EC staging, and stringent guidelines together with quality assessment are strongly urged to be adopted by other institutions.
Given the lack of well-designed clinical trials investigating the optimal surgical interventions for patients with EC, estimates of overall morbidity related to EC surgery are critical [17]. EC patients with complex underlying comorbidities are most likely to have adverse oncologic outcomes as a result of poor adherence to treatment guidelines in addition to increased surgical morbidity [51–53]. Until recently, however, the relationship between clinical risk factors and surgical outcomes had been inadequately explored. A large prospective study in 2012 reported on predictors of 30-day postoperative morbidity as classified by accordion grade (Box 18.1) and cost [17]. In this cohort, 84 % of 30-day complications were minor or moderate (accordion grade 1 or 2). An analysis of 1,369 patients showed that grade 2 or higher morbidity is independently associated with the following patient risk factors: American Society of Anesthesiologists (ASA) score >2, preoperative white blood count, history of deep venous thrombosis (DVT), minimally invasive surgery, and type of lymphadenectomy. These data were used to develop a “counseling model” that would allow the estimation of risk of operative morbidity preoperatively. Interestingly, a preoperative creatinine more than 1.5 mg/dL was a strong predictor of grade 3 or higher complications. When process of care variables were considered collectively, all previous patient-specific variables in addition to myometrial invasion >50 %, operative time, and increased surgical complexity were used to construct a “global model” (Table 18.2). This model may allow identification of interventions to reduce morbidity.
Table 18.2
Factors associated with grade 2 or higher complications within 30 days of surgery
Multivariable model of counseling factors* | Multivariable model of global factors* | |
|---|---|---|
Patient characteristics | ||
ASA class higher than 2 | 2.14 (1.65–2.78) | 1.98 (1.50–2.60) |
Preoperative WBC (×109/L)a | 2.06 (1.53–2.77) | 1.71 (1.25–2.34) |
History of DVT | 2.05 (1.28–3.28) | 2.10 (1.29–3.41) |
Surgical characteristics | ||
Type of lymphadenectomy | ||
None | Referent | Referent |
Pelvic only | 0.85 (0.54–1.34) | 1.03 (0.52–2.03) |
Paraaortic or pelvic and paraaortic | 2.34 (1.71–3.22) | 2.06 (1.12–3.79) |
Minimally invasive surgery | ||
Minimally invasive | Referent | Referent |
Vaginal only | 0.50 (0.16–1.55) | 1.46 (0.42–5.07) |
Laparotomy | 2.84 (1.45–5.54) | 4.24 (2.04–8.81) |
Tumor characteristics | ||
Myometrial invasion more than 50 % | 2.38 (1.74–3.24) | |
Process-of-care characteristics | ||
Operating time, mina | 1.85 (1.39–2.46) | |
Surgical complexityb | ||
Grade 1 | Referent | |
Grade 2 | 0.61 (0.29–1.27) | |
Grade 3 | 1.19 (0.65–2.19) | |
Grade 4 | 2.67 (1.17–6.11) | |
Box 18.1 Accordion Severity Grading System for Postoperative Complications
Severity grade | Grading criteria |
1. Mild complication | Requires only minor invasive procedures that can be done at the bedside, such as insertion of− intravenous lines, urinary catheters, and nasogastric tubes, and drainage of− wound infections. Physiotherapy and the following drugs are allowed: antiemetic, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy |
2. Moderate complication | Requires pharmacologic treatment with drugs other than those allowed for minor complications, e.g., antibiotics. Blood transfusions and total parenteral nutrition are also included |
3. Severe: invasive procedure without general anesthesia | Requires management by an endoscopic, interventional procedure or reoperation without general anesthesia |
4. Severe: operation under general anesthesia | Requires management by an operation under general anesthesia |
5. Severe: organ system failure | Organ system failure (i.e., ≥1 organ failure) |
6. Death | Postoperative death (i.e., 100-day and in-hospital death) |
Adapted from Strasberg et al. [54]
It is notable that surgical approach and use of paraaortic lymphadenectomy were strong predictors of grade 2 or higher complications in both models. Patients undergoing paraaortic lymphadenectomy were twice as likely to experience a complication, while patients requiring complex surgical procedures were also more likely (2.7 times) to experience a grade 2 or higher complication compared to patients undergoing hysterectomy and bilateral salpingo-oophorectomy only [17]. Furthermore, laparotomy was associated with a 4-fold increase in complications compared to minimally invasive surgery [17]. Of the group of patients with low-risk EC who did not require lymphadenectomy, vaginal hysterectomy was only performed in 23.3 % of patients. These patients had favorable outcomes and comparable recurrence-free and disease-specific survival rates compared to patients undergoing laparotomy or laparoscopy. In fact, no patients undergoing vaginal hysterectomy experienced disease recurrence or death secondary to disease [17]. Thus, vaginal hysterectomy was found to be the least invasive of all surgical procedure and the most cost effective procedure, and is the procedure of choice for low-risk patients (Table 18.3). These findings are in concordance with recent advances in the incorporation of minimally invasive surgery into routine gynecologic oncology practice in the USA [50]. The GOG Lap2 data demonstrated that laparoscopic staging for endometrial cancer is feasible and safe from a surgical and oncologic perspective [55]. In addition to a reduction in morbidity, minimally invasive surgery allows greater flexibility in surgical management and ease of modification of treatment planning in cases where intraoperative assessment is inconsistent with final pathology [16, 50].
Table 18.3
Intra- and postoperative factors stratified by whether lymphadenectomy was performed
Parameters | Lymphadenectomy | Total (N = 385) | P-valuea | |
|---|---|---|---|---|
No | Yes | |||
(N = 305) | (N = 80) | |||
Surgical approach. N (X) | <0.001 | |||
No laparotomy | 24 (7.9) | 5 (6.3) | 29 (7.5) | |
Laparotomy | 210 (68.9) | 75 (93.8) | 285 (74.0) | |
Vaginal only | 71 (23.3) | – | 71 (18.4) | |
OR time (minutes) | <0.001 | |||
Mean(SD) | 108.0 (52.9) | 150.6 (71.5) | 116.8 (59.8) | |
Median (IQR) | 96.0 (71.0, 130.0) | 137.0 (112.5, 170.5) | 104.0 (75.0, 143.0) | |
Estimated blood loss (ml) | <0.001 | |||
Mean(SD) | 231.6 (193.7) | 309.9 (190.7) | 247.9(195.4) | |
Median (IQR) | 200.0 (100.0, 300.0) | 250.0 (200.0, 400.0) | 200.0 (100.0, 300.0) | |
Length of hospital stay (days) | <0.001 | |||
Mean (SD) | 3.3 (2.6) | 3.7 (1.7) | 3.4 (2.4) | |
Median (IQR) | 3.0 (2.0, 4.5) | 3.0 (3.0, 4.0) | 3.0 (2.0, 4.0) | |
30-Day postoperative complicationsb, N (%) | 0.002c | |||
None | 246 (80.7) | 50 (61.5) | 296 (76.9) | |
Grade 1 | 28 (9.2) | 17 (21.3) | 45 (11.7) | |
Grade ≥2 | 31 (10.2) | 13 (16.3) | 44 (11.4) | |
Regarding cost of care, when modifiable risk factors were considered, only surgical approach was found to influence 30-day cost (Table 18.4) [17]. These data indicate that EC patients undergoing minimally invasive surgery are likely to have a lower cost-benefit ratio than patients undergoing laparotomy. However, this information is most applicable to affluent societies where surgical resources are available and affordable. Moreover, both pelvic and paraaortic lymphadenectomy were associated with increased cost of care; paraaortic lymphadenectomy was also associated with higher 30-day morbidity [16]. When low-risk patients unnecessarily undergo pelvic and or paraaortic lymphadenectomy, a significant increase in 30-day cost is incurred [17]. These initiatives to explore surgical outcomes and cost provide an opportunity to modify quality of care worldwide and further highlight the merits of selective lymphadenectomy.
Table 18.4
30-day cost of care according to accordion grading classification
Complication grade | n (%) | 30-d cost |
|---|---|---|
None | 775 [56] | $15,236 ± 5,610 |
$14,386 ($10,738–18,656) | ||
($6,466–54,638) | ||
1 | 196 [14] | $18,211 ± 6,362 |
$17,546 ($12,947–21,624) | ||
($3,189–44,030) | ||
2 | 303 [22] | $25,725 ± 12,342 |
$23,128 ($17,411–31,118) | ||
($9,843–117,571) | ||
3 | 46 [3] | $39,201 ± 19,017) |
$35,640 ($25,638–52,698) | ||
($13,099–91,662) | ||
4
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|