Materials and Methods
Study design population
This retrospective cohort study included all singleton and twin live-born neonates born between 24 0/7 and 33 6/7 weeks of gestation and admitted to level III neonatal intensive care units participating in the Canadian Neonatal Network between January 2010 and December 2014. Exclusion criteria included birthweight less than the third percentile (because fetal growth restriction has been shown to affect the impact of antenatal corticosteroids ), clinical chorioamnionitis, major congenital anomalies, incomplete information on the administration of antenatal corticosteroids, administration of more than 1 course of antenatal corticosteroids, administration of a partial course of antenatal corticosteroids, or administration of antenatal corticosteroids <24 hours or >7 days before birth.
The Canadian Neonatal Network maintains a national database of outcomes, risk factors, and practices for infants admitted to level III neonatal intensive care units in Canada. At each site, data are collected from patient charts by trained abstractors according to standard definitions and entered electronically into a customized data entry program with built-in error checks.
Approval for data collection is granted at sites by either the local Research Ethics Board or through an institutional quality improvement process for data collection and benchmarking. For this project, we obtained institutional approval from the Mount Sinai Hospital Research Ethics Board and the Canadian Neonatal Network Executive Committee.
Exposure
Neonatal outcomes were compared between twin neonates that received a complete course of antenatal corticosteroids 1–7 days before birth (Twins–ACS1–7d group) and those that did not receive antenatal corticosteroids (Twins–no ACS group). As a comparison, we performed a similar analysis in singleton neonates that received a complete course of antenatal corticosteroids 1–7 days before birth (Singletons–ACS1–7d group) and those that did not receive antenatal corticosteroids (Singletons–no ACS group). The decision to limit the exposure groups to neonates that received antenatal corticosteroids 1–7 days before birth was based on available evidence that the effect of antenatal corticosteroids in appropriate-for-gestational-age fetuses is highest when the antenatal corticosteroids are administrated within 1–7 days before birth.
Exposure to antenatal corticosteroids consisted of either betamethasone (intramuscularly, 2 doses of 12 mg 24 hours apart) or dexamethasone (intramuscularly, 4 doses of 6 mg 12 hours apart). Although the type of antenatal corticosteroids used is not documented in the Canadian Neonatal Network database, the majority of women would have received betamethasone because it is the most common type of antenatal corticosteroids used in Canada.
Outcomes
The following neonatal outcomes were evaluated: (1) mortality (death prior to discharge from neonatal intensive care units); (2) respiratory morbidity, including need for and duration of mechanical ventilation, respiratory distress syndrome, and bronchopulmonary dysplasia, defined as the requirement for oxygen at postmenstrual age of 36 weeks or at the time of transfer to a level II facility ; (3) severe neurological injury, defined as grade 3 or 4 intraventricular hemorrhage according to the criteria of Papile et al and/or periventricular leukomalacia, diagnosed by cranial ultrasound or magnetic resonance imaging or at autopsy; (4) severe retinopathy of prematurity, defined as stage 3 or higher according to the international classification of retinopathy of prematurity or retinopathy of prematurity requiring treatment; and (5) necrotizing enterocolitis, defined according to the criteria of Bell et al.
Other variables collected include infant sex, gestational age (determined from date of in vitro fertilization, first-trimester ultrasound, last menstrual period, obstetric estimate, and pediatric estimate, in that order), birthweight, outborn admission, Apgar score at 5 minutes, maternal demographics, and maternal hypertension.
Statistical analysis
Maternal characteristics and neonatal outcomes were compared between the ACS1–7d and no ACS groups using the Student t test and the χ 2 test for continuous and categorical variables, respectively. A multivariable logistic regression analysis was used to assess the association between the administration of antenatal corticosteroids 1–7 days before birth and adverse neonatal outcomes after adjustment for variables that were found to be different between the 2 study groups and those variable for which there is biological rationale to consider them as potential confounders.
The strategy described in the previous text regarding which variables would be included in the regression model was determined during the design phase of the study. These models were fitted with generalized estimating equation to account for correlation within a pair of twins from the same mother. Neonates that were not exposed to antenatal corticosteroids were used as a reference groups. Separate analyses were performed for twin and singleton neonates.
Adjusted odds ratios and 95% confidence intervals were calculated for twins and singletons and were compared between the 2 groups to identify the differential effects. The odds ratio for the association of antenatal corticosteroids with neonatal outcome in the twins and singletons groups were compared using the following approach: first, the SE of each odds ratio was obtained from the corresponding adjusted regression models. Then the combined SE for the difference between the 2 odds ratios was calculated (defined as the square root of ([SE1] ∧ 2 + [SE2] ∧ 2]).
The Z statistic (defined as the estimate of the difference divided by the combined SE) was calculated, and the associated P value was reported. The significance of statistical tests was evaluated using 2-sided P values at the 5% significance level. Data were analyzed using the statistical package SAS (version 9.3; SAS Institute, Cary, NC).
Results
Characteristics of the study groups
A total of 25,536 neonates born at a gestational age of 24 0/7 to 33 6/7 weeks were identified during the study period from the Canadian Neonatal Network database, of which 9466 were eligible for the study. Of the 2516 twin neonates, 1758 (69.9%) received antenatal corticosteroids within 1–7 days before birth and 758 (30.1%) did not receive antenatal corticosteroids ( Figure ). Of the 6950 singletons, 4638 (66.7%) received antenatal corticosteroids within 1–7 days before birth, whereas 2312 (33.3%) were not exposed to antenatal corticosteroids ( Figure ).
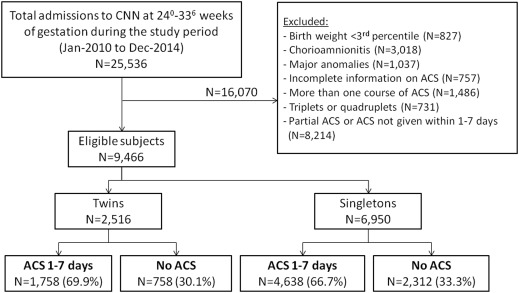
The characteristics of the study groups are presented in Table 1 . In both twin and singleton pregnancies, women who received antenatal corticosteroids were more likely to be nulliparous and experience hypertension, less likely to deliver outside a tertiary center (outborn), and had a lower gestational age at birth compared with women who did not receive antenatal corticosteroids ( Table 1 ). In the twins group, women who received antenatal corticosteroids were older than women who did not. Among singletons, there were small but significant differences in the proportion of male fetuses and the rate of cesarean delivery between women who received and did not receive antenatal corticosteroids ( Table 1 ).
| Characteristics | Twins | Singletons | ||||
|---|---|---|---|---|---|---|
| ACS within 1–7 d (n = 1068) a | No ACS (n = 497) a | P value | ACS within 1–7 d (n = 4638) | No ACS (n = 2312) | P value | |
| Maternal age >35 y | 193 (25%) | 59 (19%) | .046 | 660 (19%) | 247 (19%) | .72 |
| Nulliparity | 625 (62%) | 226 (47%) | < .001 | 2365 (53%) | 989 (44%) | < .001 |
| Gestational diabetes | 105 (10%) | 41 (9%) | .40 | 539 (12%) | 261 (12%) | .98 |
| Maternal hypertension | 185 (18%) | 43 (9%) | < .001 | 1466 (32%) | 322 (15%) | < .001 |
| Outborn status | 59 (6%) | 154 (31%) | < .001 | 307 (7%) | 935 (40%) | < .001 |
| Cesarean birth | 720 (67%) | 316 (64%) | .15 | 2569 (55%) | 1096 (48%) | < .001 |
| Gestational age, wks, mean (SD) | 29.8 (2.7) | 30.6 (2.7) | < .001 | 29.5 (2.7) | 30.4 (2.6) | < .001 |
| Gestational age <32 0/7 wks | 710 (66%) | 227 (46%) | < .001 | 3280 (71%) | 1219 (53%) | < .001 |
| Antenatal MgSO 4 for neuroprotection | 418 (40%) | 39 (8%) | < .01 | 2081 (46%) | 207 (10%) | < .001 |
Neonatal outcomes
Unadjusted estimates of neonatal outcomes are presented in Table 2 . Twin and singleton neonates born to women who received antenatal corticosteroids had lower rates of mortality, mechanical ventilation, and severe neurological injury as compared with neonates born to women who did not receive antenatal corticosteroids ( Table 2 ). Among twins, no differences were observed between the ACS1-7d and no ACS groups with respect to the rate of respiratory distress syndrome, bronchopulmonary dysplasia, and necrotizing enterocolitis. Among singletons, neonates exposed to antenatal corticosteroids 1–7 days prior to birth had a lower rate of respiratory distress syndrome and a higher rate of bronchopulmonary dysplasia and necrotizing enterocolitis in comparison with those who did not receive antenatal corticosteroids ( Table 2 ).
| Variables | Twins | Singletons | ||||
|---|---|---|---|---|---|---|
| ACS within 1–7 d (n = 1758) a | No ACS (n = 758) a | P value | ACS within 1–7 d (n = 4638) | No ACS (n = 2312) | P value | |
| Male sex | 916 (52%) | 407 (54%) | .46 | 2546 (55%) | 1333 (58%) | .03 |
| Growth parameters at birth | ||||||
| Birthweight, g, mean (SD) | 1458 (456) | 1646 (482) | < .001 | 1398 (501) | 1665 (569) | < .001 |
| Birthweight (Z score), mean (SD) b | –0.07 (0.87) | 0.29 (0.92) | < .001 | –0.18 (0.96) | 0.33 (1.09) | < .001 |
| Birthweight <10th percentile b | 139 (8%) | 38 (5%) | .009 | 511 (11%) | 155 (7%) | < .001 |
| Head circumference, cm, mean (SD) | 28.1 (2.9) | 29.1 (2.9) | < .001 | 27.3 (3.1) | 28.7 (3.1) | < .001 |
| Head circumference (Z score), mean (SD) b | 0.07 (0.98) | 0.40 (0.98) | < .001 | –0.19 (1.04) | 0.26 (1.03) | < .001 |
| Neonatal outcomes | ||||||
| Mortality | 57 (3%) | 42 (6%) | .007 | 211 (5%) | 171 (7%) | < .001 |
| Mechanical ventilation | 617 (35%) | 314 (41%) | .003 | 1870 (40%) | 1088 (47%) | < .001 |
| Mechanical ventilation days, median (IQR) | 0 (0-2) | 0 (0-2) | .09 | 0 (0-3) | 0 (0-3) | .004 |
| Respiratory distress syndrome | 807 (46%) | 374 (50%) | .10 | 2254 (49%) | 1183 (52%) | .009 |
| Bronchopulmonary dysplasia | 172 (10%) | 60 (8%) | .17 | 597 (14%) | 215 (10%) | < .001 |
| Severe neurological injury c | 74 (6%) | 42 (9%) | .02 | 230 (7%) | 201 (14%) | < .001 |
| Retinopathy of prematurity ≥stage 3 | 40 (7%) | 6 (4%) | .14 | 130 (7%) | 38 (7%) | .77 |
| Necrotizing enterocolitis (stage 2 or 3) | 71 (4%) | 22 (3%) | .16 | 190 (4%) | 70 (3%) | .03 |
a n refers to the number of neonates in each group
b Calculated using mean and SD from the whole Canadian neonate population of 24–34 weeks of gestational age
c Defined as grade 3 or 4 intraventricular hemorrhage and/or periventricular leukomalacia.
Both twin and singleton neonates in the ACS1–7d groups had a lower mean birthweight Z-score, a lower head circumference Z-score, and a higher birthweight below the 10th percentile for gestational age as compared with those not exposed to antenatal corticosteroids ( Table 2 ); however, they were born at a lower gestational age in both populations.
Association between antenatal corticosteroids and neonatal outcomes in twins and singletons: multivariable analysis
A multivariable logistic regression analysis was performed to examine the association between antenatal corticosteroids and various neonatal outcomes, which are reported in Table 3 . Analyses were adjusted for gestational age, maternal hypertension, outborn status, parity, sex, small-for-gestational-age status, and cesarean birth. Using newborns who did not receive antenatal corticosteroids as a reference, the administration of antenatal corticosteroids 1–7 days before birth was associated with a lower odds of neonatal mortality, mechanical ventilation, respiratory distress syndrome, and severe neurological morbidity in both twins and singletons, and the corresponding adjusted odds ratios were similar between the 2 groups (0.42 vs 0.38, P = .69; 0.47 vs 0.47, P = .93; 0.53 vs 0.54, P = .89; and 0.50 vs 0.45, P = .75, respectively) ( Table 3 ). There was no association between the administration of antenatal corticosteroids and the odds of bronchopulmonary dysplasia, severe retinopathy, or necrotizing enterocolitis in either twins or singletons.
| Outcome | ACS administrated 1–7 d before birth compared with no ACS | ||
|---|---|---|---|
| Twin pregnancies AOR (95% CI) a , b | Singleton pregnancies AOR (95% CI) a | P value c | |
| Mortality | 0.42 (0.24–0.76) | 0.38 (0.28–0.50) | .69 |
| Mechanical ventilation | 0.47 (0.35–0.63) | 0.47 (0.41–0.55) | .93 |
| Respiratory distress syndrome | 0.53 (0.40–0.69) | 0.54 (0.47–0.62) | .89 |
| Bronchopulmonary dysplasia | 0.69 (0.42–1.11) | 0.80 (0.63–1.02) | .57 |
| Severe neurological injury d | 0.50 (0.30–0.83) | 0.45 (0.34–0.59) | .75 |
| Retinopathy of prematurity ≥stage 3 | 2.37 (0.74–7.59) | 0.79 (0.47–1.32) | .09 |
| Necrotizing enterocolitis (stage 2 or 3) | 1.24 (0.65–2.38) | 1.07 (0.78–1.47) | .69 |
a Model adjusted for gestational age, sex, hypertension, outborn status, small for gestational age (<10 th percentile), parity, and cesarean birth
b Modeled using logistic regression fitted with generalized estimating equation to account for correlation within a pair of twins from the same mother
c Refers to the comparison of odds ratios in twin vs singleton pregnancies
d Defined as grade 3 or 4 intraventricular hemorrhage and/or periventricular leukomalacia.
Results
Characteristics of the study groups
A total of 25,536 neonates born at a gestational age of 24 0/7 to 33 6/7 weeks were identified during the study period from the Canadian Neonatal Network database, of which 9466 were eligible for the study. Of the 2516 twin neonates, 1758 (69.9%) received antenatal corticosteroids within 1–7 days before birth and 758 (30.1%) did not receive antenatal corticosteroids ( Figure ). Of the 6950 singletons, 4638 (66.7%) received antenatal corticosteroids within 1–7 days before birth, whereas 2312 (33.3%) were not exposed to antenatal corticosteroids ( Figure ).
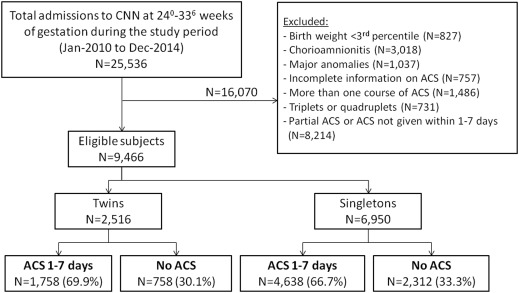
The characteristics of the study groups are presented in Table 1 . In both twin and singleton pregnancies, women who received antenatal corticosteroids were more likely to be nulliparous and experience hypertension, less likely to deliver outside a tertiary center (outborn), and had a lower gestational age at birth compared with women who did not receive antenatal corticosteroids ( Table 1 ). In the twins group, women who received antenatal corticosteroids were older than women who did not. Among singletons, there were small but significant differences in the proportion of male fetuses and the rate of cesarean delivery between women who received and did not receive antenatal corticosteroids ( Table 1 ).



