The Patient with Single Ventricle
Jacqueline Kreutzer
Single ventricle or univentricular heart refers to a limited number of congenital heart lesions from a strict anatomic perspective. Anatomically, single ventricle lesions can be either a single left ventricle, due to agenesis of the right ventricular inlet (there is often a small component of the right ventricular outlet present), or a single right ventricle, secondary to agenesis of the left ventricle.1,2 In both, the atria drain through one or two atrioventricular valves into the only ventricular chamber (Fig. 498-1). These lesions are rare, and do not include common lesions such as tricuspid atresia and hypoplastic left-heart syndrome.
In practice the concept of “single ventricle” expands beyond the strict anatomic definition, and refers to all congenital heart lesions that share a single ventricle physiology, regardless of the underlying structural variant.3,4 This expanded functional definition includes all those lesions in which the patient lacks a second ventricle that can independently support the pulmonary circulation. In most such lesions, components of a second ventricle exist. 
Thus, the single ventricle from a physiologic perspective includes patients with
• single right ventricle
• single left ventricle
• unbalanced complete atrioventricular (AV) canal (a common AV valve is more aligned to one ventricle than the other, typically associated with asymmetry in the development of the two ventricular chambers)
• tricuspid atresia (eFig. 498.1  )
)
• hypoplastic tricsupid valve and right ventricle, frequently with intact ventricular septum and pulmonary atresia
• mitral atresia
• hypoplastic left heart syndrome and its variants (eFig. 498.2  )
)
PATHOPHYSIOLOGY
Overall, patients with single ventricle preoperatively tend to have complete mixing of pulmonary and systemic venous returns within the heart (Fig. 498-2). Blood leaving the heart goes both to the pulmonary and systemic circulations; therefore, the aortic and pulmonary oxygen saturations are usually equal but sometimes streaming of blood within the heart causes differences in aortic and pulmonary oxygen saturations. The single ventricle is volume overloaded, as it supplies both circulations. This parallel circulation is relatively inefficient because some previously oxygenated blood from the lungs returns to the pulmonary circulation and some systemic venous blood is shunted into the systemic arterial circulation (Fig. 498-2, eFig. 498.4  ).3
).3
The balance between the systemic and the pulmonary circulations depends on multiple factors, including anatomic obstruction as well as relative vascular resistances and perfusion pressure. Ideally, the relationship between the pulmonary and systemic blood flows (Qp/Qs) should be kept at least at 1:1, which typically is achieved in the presence of complete mixing when the systemic arterial oxygen saturation is around 75%, in the absence of lung disease and with a normal cardiac output. Some cardiologists recommend maintaining aortic oxygen saturation over 85% with a QP/QS of 1.5 to 2, to improve growth potential.
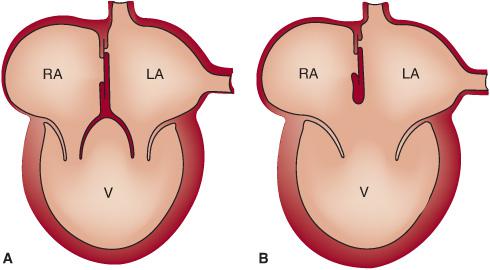
FIGURE 498-1. Diagram illustrates anatomy of double inlet single ventricle (A) and common inlet single ventricle (B). (Source: Modified from Schultz AH, Kreutzer J. Cyanotic heart disease. In: Vetter VL, ed. Pediatric Cardiology. The Requisites in Pediatrics. Philadelphia, PA: Mosby Elsevier; 2006:51-78.)
In the absence of obstruction at any level, the relationship between pulmonary and systemic blood flow depends on the relative resistances of the two vascular beds. At birth, Qp/Qs is only modestly increased as pulmonary vascular resistance falls from in utero levels, so that systemic arterial oxygen saturation is generally in the mid 70s. As pulmonary vascular resistance decreases in the first few weeks of life, pulmonary blood flow and systemic arterial oxgyen saturation increase greatly because the vascular resistance of the pulmonary vascular bed is much lower than that of the systemic bed. Saturations greater than 85% are associated with such high pulmonary blood flow and in some of these patients heart failure may develop, with respiratory distress, diaphoresis, and failure to thrive. Providing supplemental oxygen to infants with single ventricle to increase saturations when the oxygen levels are above 80% can be detrimental, as oxygen acts as a pulmonary vasodilator, decreasing pulmonary vascular resistance, increasing the pulmonary blood flow, altering the Qp/Qs relationship such that the systemic output decreases.3
However, many patients with single ventricle physiology have various levels of obstruction within the heart and great vessels, so that Qp/Qs depends on factors other than relative vascular resistances. The levels of obstruction include
• Pulmonary or subpulmonary stenosis or atresia: This may lead to a marked decrease in systemic arterial oxygen saturation, as the ductus arteriosus closes over the first hours of life. The degree of cyanosis is determined by the severity of the obstruction. In patients with severe pulmonary stenosis or pulmonary atresia, severe cyanosis can occur rapidly, and is a life-threatening event. In that situation, the infant is considered to be “ductal dependent.” Prostaglandin E1 is started immediately to reverse the problem. The typical hemodynamic status once the PDA is opened is illustrated in eFigure 498.6  .
.
• Systemic (aortic) obstruction: This can be below the aortic valve (subaortic stenosis), at the valve, in the aortic arch (hypoplasia or aortic arch interruption), or in the aortic isthmus between the left subclavian artery and ductus arteriosus (coarctation of the aorta). When the obstruction is critical, newborns are ductal dependent, meaning that patency of the ductus arteriosus is necessary to maintain adequate systemic blood flow.
• Atrioventricular obstruction: With a stenotic or atretic atrioventricular valve, restriction of flow between the two atria can be very deleterious. In the hypoplastic left heart syndrome, for example, restriction at the foramen ovale causes severe left atrial and pulmonary venous hypertension. 
NATURAL HISTORY
The vast majority of the patients with single ventricle variants develop symptoms in the newborn period and early infancy. Patients with ductal dependent lesions need emergency intervention. Those with increased pulmonary blood flow may show signs of heart failure as the pulmonary vascular resistance drops, whereas those with decreased pulmonary blood flow have severe cyanosis. If not treated, death occurs in infancy in more than 50% of patients,12-14 more than half occurring during the neonatal period.
Patients with increased pulmonary blood flow may develop irreversible pulmonary vascular changes after the first year of life, leading to a decrease in the Qp/Qs. As pulmonary blood flow decreases the symptoms of heart failure resolve, but progressively severe cyanosis appears. Typical complicating features related to cyanosis and polycythemia include stroke, renal dysfunction, scoliosis, and endocarditis. Patients eventually develop progressive ventricular dysfunction, arrhythmias, and worsening heart failure. 
PHYSICAL EXAMINATION
Patients with single ventricle have variable physical examinations, depending on the presence or absence of associated pathology, particularly pulmonary or systemic obstruction.15
Pulmonary stenosis (decreased pulmonary blood flow): Variable cyanosis is present. On auscultation, there is a systolic ejection murmur of intensity and duration directly correlating with the amount of pulmonary blood flow, and inversely correlated with the severity of the pulmonary stenosis and level of cyanosis. The absence of a systolic ejection murmur may suggest pulmonary stenosis. A continuous murmur from a patent ductus or aortopulmonary collateral vessels may be present.
No pulmonary stenosis (increased pulmonary blood flow): With no pulmonary stenosis, patients tend to present with heart failure due to increased pulmonary blood flow, manifested by respiratory distress, diaphoresis, and poor weight gain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


