Disappearance of the anterior vascular capsule of the lens is also helpful in determining gestational age. Until 27–28 weeks’ gestation, the lens capsule is covered by vessels; by 34 weeks, this vascular plexus is completely atrophied. Foot length, from the heel to the tip of the longest toe, also correlates with gestational age in appropriately grown infants. The foot measures 4.5 cm at 25 weeks’ gestation and increases 0.25 cm/wk until term.
If the physical examination indicates a gestational age within 2 weeks of that predicted by the obstetric dates, the gestational age is as assigned by the obstetric dating. Birth weight and gestational age are plotted on standard grids (Figure 2–1) to determine whether the birth weight is appropriate for gestational age (AGA), small for gestational age (SGA, also known as intrauterine growth restriction or IUGR), or large for gestational age (LGA). Birth weight for gestational age in normal neonates varies with race, maternal nutrition, access to obstetric care, and environmental factors such as altitude, smoking, and drug and alcohol use. Whenever possible, standards for newborn weight and gestational age based on local or regional data should be used. Birth weight related to gestational age is a screening tool that should be supplemented by clinical data when entertaining a diagnosis of IUGR or excessive fetal growth. These data include the infant’s physical examination and other factors such as parental size and the birth weight–gestational age of siblings.
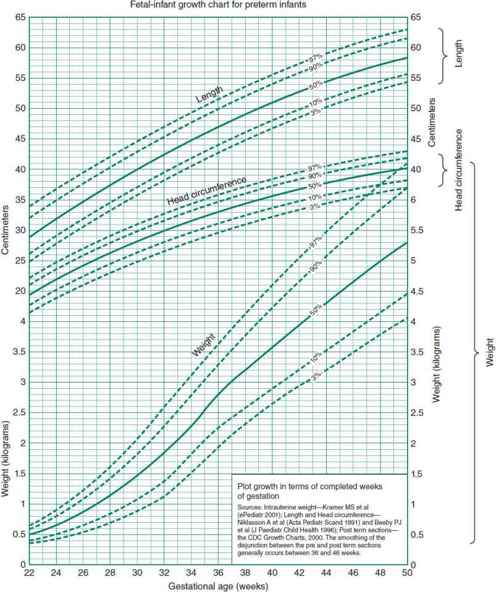
 Figure 2–1. Fetal-infant growth chart for weight, length, and head circumference. Sources: intrauterine weight, length and head circumference, postterm sections—CDC growth charts 2000. URL. http://www.biomedcentral.com/1471-2431/3/13. (Reproduced with permission from Fenton TR: A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format, BMC Pediatr. 2003 Dec 16;3:13.)
Figure 2–1. Fetal-infant growth chart for weight, length, and head circumference. Sources: intrauterine weight, length and head circumference, postterm sections—CDC growth charts 2000. URL. http://www.biomedcentral.com/1471-2431/3/13. (Reproduced with permission from Fenton TR: A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format, BMC Pediatr. 2003 Dec 16;3:13.)
An important distinction, particularly in SGA infants, is whether a growth disorder is symmetrical (weight, length, and occipitofrontal circumference [OFC] all ≤ 10%) or asymmetrical (only weight ≤ 10%). Asymmetrical growth restriction implies a problem late in pregnancy, such as pregnancy-induced hypertension or placental insufficiency. Symmetrical growth restriction implies an event of early pregnancy: chromosomal abnormality, drug or alcohol use, or congenital viral infections (Table 2–2). In general, the outlook for normal growth and development is better in asymmetrically growth-restricted infants whose intrauterine brain growth has been spared.
Table 2–2. Causes of variations in neonatal size in relation to gestational age.

The fact that SGA infants have fewer problems (such as respiratory distress syndrome) than AGA infants of the same birth weight but a lower gestational age has led to the misconception that SGA infants have accelerated maturation. SGA infants, when compared with AGA infants of the same gestational age, actually have increased morbidity and mortality rates.
Knowledge of birth weight in relation to gestational age allows anticipation of some neonatal problems. LGA infants are at risk for birth trauma; LGA infants of diabetic mothers are also at risk for hypoglycemia, polycythemia, congenital anomalies, cardiomyopathy, hyperbilirubinemia, and hypocalcemia. SGA infants are at risk for fetal distress during labor and delivery, polycythemia, hypoglycemia, and hypocalcemia.
American Academy of Pediatrics Committee on Fetus and Newborn: Levels of neonatal care. Pediatrics 2012;130:587 [PMID: 22926177].
Blumenfeld Y: First trimester screening for fetal aneuploidy. NeoReviews 2012;13:e4.
Gardosi J: Clinical strategies for improving the detection of fetal growth restriction. Clin Perinatol 2011;38:21 [PMID: 21353087].
Maulik D et al: Umbilical artery Doppler in the assessment of fetal growth restriction. Clin Perinatol 2011;38:65 [PMID: 21353090].
Rosenberg A: The IUGR newborn. Semin Perinatol 2008;32:219 [PMID: 18482625].
EXAMINATION AT BIRTH
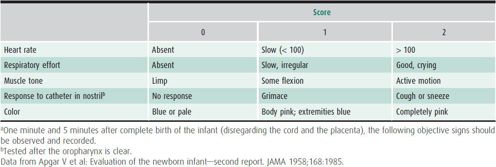
The extent of the newborn physical examination depends on the condition of the infant and the setting. Examination in the delivery room consists largely of observation plus auscultation of the chest and inspection for congenital anomalies and birth trauma. Major congenital anomalies occur in 1.5% of live births and account for 20%–25% of perinatal and neonatal deaths. Because infants are physically stressed during parturition, the delivery room examination should not be extensive. The Apgar score (Table 2–3) should be recorded at 1 and 5 minutes of age. In severely depressed infants, scores can be recorded out to 20 minutes. Although the 1- and 5-minute Apgar scores have almost no predictive value for long-term outcome, serial scores provide a useful description of the severity of perinatal depression and the response to resuscitative efforts.
Table 2–3. Infant evaluation at birth—Apgar score.a
Skin color is an indicator of cardiac output because of the normal high blood flow to the skin. Stress that triggers a catecholamine response redirects cardiac output away from the skin to preserve oxygen delivery to more critical organs. Cyanosis and pallor are thus two useful signs suggestive of inadequate cardiac output.
Skeletal examination at delivery serves to detect obvious congenital anomalies and to identify birth trauma, particularly in LGA infants or those born after a protracted second stage of labor where a fractured clavicle or humerus might be found.
The number of umbilical cord vessels should be determined. Normally, there are two arteries and one vein. In 1% of deliveries (5%–6% of twin deliveries), the cord has only one artery and one vein. This minor anomaly slightly increases the risk of associated defects. The placenta should be examined at delivery. Small placentas are always associated with small infants. The placental examination includes identification of membranes and vessels (particularly in multiple gestations) as well as placental infarcts or clots (placental abruption) on the maternal side.
EXAMINATION IN THE NURSERY
The purpose of the newborn examination is to identify abnormalities or anomalies that might impact the infant’s well-being, and to evaluate for any acute illness or difficulty in the transition from intrauterine to extrauterine life. The examiner should have warm hands and a gentle approach. Start with observation, then auscultation of the chest, and then palpation of the abdomen. Examination of the eyes, ears, throat, and hips should be performed last, as these maneuvers are most disturbing to the infant. The heart rate should range from 120 to 160 beats/min and the respiratory rate from 30 to 60 breaths/min. Systolic blood pressure on day 1 ranges from 50 to 70 mm Hg and increases steadily during the first week of life. Blood pressure is influenced more significantly by perinatal asphyxia and mechanical ventilation than it is by gestational age. An irregularly irregular heart rate, usually caused by premature atrial contractions, is common, benign, and usually resolves in the first days of life.
Approximately 15%–20% of healthy newborns have one minor anomaly (a common variant that would not impact the infant’s well-being; eg, a unilateral transverse palmar [simian] crease, or a single umbilical artery). Those with a minor anomaly have a 3% risk of an associated major anomaly. Approximately 0.8% of newborns have two minor anomalies, and 0.5% have three or more, with a risk of 10% and 20%, respectively, of also having a major malformation. Other common minor anomalies requiring no special investigation in healthy infants include preauricular pits, a shallow sacral dimple without other cutaneous abnormality within 2.5 cm of the anus, and three or fewer café au lait spots in a white infant or five or fewer in an African-American infant.
Skin
Observe for bruising, petechiae (common over the presenting part), meconium staining, and jaundice. Visible jaundice in the first 24 hours is never normal, and generally indicates either a hemolytic process, or a congenital hepatitis, either of which requires further evaluation. Peripheral cyanosis is commonly present when the extremities are cool or the infant is polycythemic. Generalized cyanosis merits immediate evaluation. Pallor may be caused by acute or chronic blood loss or by acidosis. In dark-skinned infants, pallor and cyanosis should be assessed in the lips, mouth, and nail beds. Plethora suggests polycythemia. Note the presence of vernix caseosa (a whitish, greasy material covering the body that decreases as term approaches) and lanugo (the fine hair covering the preterm infant’s skin). Dry skin with cracking and peeling of the superficial layers is common in postterm infants. Edema may be generalized (hydrops) or localized (eg, on the dorsum of the feet in Turner syndrome). Check for birthmarks such as capillary hemangiomas (lower occiput, eyelids, and forehead) and mongolian spots (bluish-black pigmentation over the back and buttocks). There are many benign skin eruptions such as milia, miliaria, erythema toxicum, and pustular melanosis that are present in the newborn period, but more serious conditions may be indicated by blistering or erosive lesions. See Chapter 15 for a more in-depth description of these conditions.
Head
Check for cephalohematoma (a swelling over one or both parietal bones that is contained within suture lines) and caput succedaneum (edema of the scalp over the presenting part that crosses suture lines). Subgaleal hemorrhages (beneath the scalp) are uncommon but can cause extensive blood loss into this large potential space, resulting in hypovolemic shock. Skull fractures may be linear or depressed and may be associated with cephalohematoma. Check for the presence and size of the fontanelles. The anterior fontanelle varies from 1 to 4 cm in any direction; the posterior fontanelle should be less than 1 cm. A third fontanelle is a bony defect along the sagittal suture in the parietal bones and may be seen in syndromes, such as trisomy 21. Sutures should be freely mobile, but are often overriding just after birth. Craniosynostosis, a prematurely fused suture causing an abnormal cranial shape, is more easily diagnosed a few days or more after birth.
Face
Unusual faces may be associated with a specific syndrome. Bruising from birth trauma (especially with face presentation) and forceps application should be identified. Face presentation may cause soft tissue swelling around the nose and mouth and significant facial distortion. Facial nerve palsy is most obvious during crying; the unaffected side of the mouth moves normally, giving an asymmetric grimace.
Eyes
Subconjunctival hemorrhages are a frequent result of birth trauma. Less commonly, a corneal tear (presenting as a clouded cornea), or a hyphema (a layering of blood in the anterior chamber of the eye) may occur. Ophthalmologic consultation is indicated in such cases. Extraocular movements should be assessed. Occasional uncoordinated eye movements are common, but persistent irregular movements are abnormal. The iris should be inspected for abnormalities such as speckling (Brushfield spots seen in trisomy 21) and colobomas. Retinal red reflexes should be present and symmetrical. Dark spots, unilateral blunted red reflex, absent reflex, or a white reflex all require ophthalmologic evaluation. Leukocoria can be caused by glaucoma (cloudy cornea), cataract, or tumor (retinoblastoma). Infants with suspected or known congenital viral infection should have a retinoscopic examination with pupils dilated to look for chorioretinitis.
Nose
Examine the nose for size and shape. In-utero compression can cause deformities. Because infants younger than 1 month of age are obligate nose breathers, any nasal obstruction (eg, bilateral choanal atresia or stenosis) can cause respiratory distress. Unilateral choanal atresia can be diagnosed by occluding each naris, although patency is best checked by holding a cold metal surface (eg, a chilled scissor) under the nose, and observing the fog from both nares on the metal. Purulent nasal discharge at birth suggests congenital syphilis (“snuffles”).
Ears
Malformed or malpositioned (low-set or posteriorly rotated) ears are often associated with other congenital anomalies. The tympanic membranes should be visualized. Preauricular pits and tags are common minor variants, and may be familial. Any external ear abnormality may be associated with hearing loss.
Mouth
Epithelial (Epstein) pearls are benign retention cysts along the gum margins and at the junction of the hard and soft palates. Natal teeth may be present and sometimes must be removed to prevent their aspiration. Check the integrity and shape of the palate for clefts and other abnormalities. A small mandible and tongue with cleft palate is seen with Pierre-Robin syndrome and can present as respiratory difficulty, as the tongue occludes the airway; prone positioning can be beneficial. A prominent tongue can be seen in trisomy 21 and Beckwith-Wiedemann syndrome. Excessive oral secretions suggest esophageal atresia or a swallowing disorder.
Neck
Redundant neck skin or webbing, with a low posterior hairline, is seen in Turner syndrome. Cervical sinus tracts may be seen as remnants of branchial clefts. Check for masses: mid-line (thyroglossal duct cysts), anterior to the sternocleidomastoid (branchial cleft cysts), within the sternocleidomastoid (hematoma and torticollis), and posterior to the sternocleidomastoid (cystic hygroma).
Chest & Lungs
Check for fractured clavicles (crepitus, bruising, and tenderness). Increased anteroposterior diameter (barrel chest) can be seen with aspiration syndromes. Check air entry bilaterally and the position of the mediastinum by locating the point of maximum cardiac impulse and assessment of heart tones. Decreased breath sounds with respiratory distress and a shift in the heart tones suggest pneumothorax (tension) or a space-occupying lesion (eg, diaphragmatic hernia). Pneumomediastinum causes muffled heart sounds. Expiratory grunting and decreased air entry are observed in hyaline membrane disease. Rales are not of clinical significance at this age.
Heart
Cardiac murmurs are common in the first hours and are most often benign; conversely, severe congenital heart disease in the newborn infant may be present with no murmur at all. The two most common presentations of heart disease in the newborn infant are (1) cyanosis and (2) congestive heart failure with abnormalities of pulses and perfusion. In hypoplastic left heart and critical aortic stenosis, pulses are diminished at all sites. In aortic coarctation and interrupted aortic arch, pulses are diminished in the lower extremities.
Abdomen
Check for tenderness, distention, and bowel sounds. If polyhydramnios was present or excessive oral secretions are noted, pass a soft catheter into the stomach to rule out esophageal atresia. Most abdominal masses in the newborn infant are associated with kidney disorders (eg, multicystic or dysplastic, and hydronephrosis). When the abdomen is relaxed, normal kidneys may be felt but are not prominent. A markedly scaphoid abdomen plus respiratory distress suggests diaphragmatic hernia. Absence of abdominal musculature (prune belly syndrome) may occur in association with renal abnormalities. The liver and spleen are superficial in the neonate and can be felt with light palpation. A distended bladder may be seen as well as palpated above the pubic symphysis.
Genitalia & Anus
Male and female genitals show characteristics according to gestational age (see Table 2–1). In the female infant during the first few days, a whitish vaginal discharge with or without blood is normal. Check the patency and location of the anus.
Skeleton
Check for obvious anomalies such as absence of a bone, club-foot, fusion or webbing of digits, and extra digits. Examine for hip dislocation by attempting to dislocate the femur posteriorly and then abducting the legs to relocate the femur noting a clunk as the femoral head relocates. Look for extremity fractures and for palsies (especially brachial plexus injuries) and evidence of spinal deformities (eg, scoliosis, cysts, sinuses, myelomeningocele). Arthrogryposis (multiple joint contractures) results from chronic limitation of movement in utero that may result from lack of amniotic fluid or from congenital neuromuscular disease.
Neurologic Examination
Normal newborns have reflexes that facilitate survival (eg, rooting and sucking reflexes), and sensory abilities (eg, hearing and smelling) that allow them to recognize their mother soon after birth. Although the retina is well developed at birth, visual acuity is poor (20/400) because of a relatively immobile lens. Acuity improves rapidly over the first 6 months, with fixation and tracking becoming well developed by 2 months.
Observe the newborn’s resting tone. Normal term newborns should exhibit flexion of the upper and lower extremities and symmetrical spontaneous movements. Extension of the extremities should result in spontaneous recoil to the flexed position. Assess the character of the cry; a high-pitched cry with or without hypotonia may indicate disease of the central nervous system (CNS) such as hemorrhage or infection, a congenital neuromuscular disorder, or systemic disease. Check the following newborn reflexes:
1. Sucking reflex: The newborn sucks in response to a nipple in the mouth; observed by 14 weeks’ gestation.
2. Rooting reflex: Head turns to the side of a facial stimulus, present by 28 weeks’ gestation.
3. Traction response: The infant is pulled by the arms to a sitting position. Initially, the head lags, then with active flexion, comes to the midline briefly before falling forward.
4. Palmar grasp: Evident with the placement of the examiner’s finger in the newborn’s palm; develops by 28 weeks’ gestation and disappears by age 4 months.
5. Deep tendon reflexes: A few beats of ankle clonus and an upgoing Babinski reflex may be normal.
6. Moro (startle) reflex: Hold the infant supine while supporting the head. Allow the head to drop 1–2 cm suddenly. The arms will abduct at the shoulder and extend at the elbow with spreading of the fingers. Adduction with flexion will follow. This reflex develops by 28 weeks’ gestation (incomplete) and disappears by age 3 months.
7. Tonic neck reflex: Turn the infant’s head to one side; the arm and leg on that side will extend while the opposite arm and leg flex (“fencing position”). This reflex disappears by age 8 months.
Ahamd R-CS et al: Blisters and erosions in the neonate. NeoReviews 2011;12:e453.
Holmes LB: Common Malformations. Oxford Press; New York City, 2012.
Kanada KN et al: A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr 2012;161:240 [PMID: 22497908].
Nemeth BA, Narotam V: Developmental dysplasia of the hip. Pediatr Rev 2012;33:553 [PMID: 23204397].
Ramasubramanian A, Johnston S: Neonatal eye disorders requiring ophthalmology consultation. NeoReviews 2011;12:e216.
Zywicke HA, Rozzelle CJ: Sacral dimples. Pediatr Rev 2011;32:109 [PMID: 21364014].
CARE OF THE WELL NEONATE
The primary responsibility of the Level 1 nursery is care of the well neonate—promoting mother-infant bonding, establishing feeding, and teaching the basics of newborn care. Staff must monitor infants for signs and symptoms of illness, including temperature instability, change in activity, refusal to feed, pallor, cyanosis, early or excessive jaundice, tachypnea, respiratory distress, delayed (beyond 24 hours) first stool or first void, and bilious vomiting. Several preventive measures are routine in the normal newborn nursery.
Prophylactic erythromycin ointment is applied to the eyes within 1 hour of birth to prevent gonococcal ophthalmia. Vitamin K (1 mg) is given intramuscularly or subcutaneously within 4 hours of birth to prevent hemorrhagic disease of the newborn.
All infants should receive hepatitis B vaccine. Both hepatitis B vaccine and hepatitis B immune globulin (HBIG) are administered if the mother is positive for hepatitis B surface antigen (HBsAg). If maternal HBsAg status is unknown, vaccine should be given before 12 hours of age, maternal blood should be tested for HBsAg, and HBIG should be given to the neonate before 7 days of age if the test is positive.
Cord blood is collected from all infants at birth and can be used for blood typing and Coombs testing if the mother is type O or Rh-negative to help assess the risk for development of jaundice.
Bedside glucose testing should be performed in infants at risk for hypoglycemia (infants of diabetic mothers, preterm, SGA, LGA, or stressed infants). Values below 45 mg/dL should be confirmed by laboratory blood glucose testing and treated. Hematocrit should be measured at age 3–6 hours in infants at risk for or those who have symptoms of polycythemia or anemia (see section on Hematologic Disorders).
State-sponsored newborn genetic screens (for inborn errors of metabolism such as phenylketonuria [PKU], galactosemia, sickle cell disease, hypothyroidism, congenital adrenal hyperplasia, and cystic fibrosis) are performed prior to discharge, after 24–48 hours of age if possible. In many states, a repeat test is required at 8–14 days of age because the PKU test may be falsely negative when obtained before 48 hours of age. Not all state-mandated screens include the same panel of diseases. The most recent additions include an expanded screen that tests for other inborn errors of metabolism such as fatty acid oxidation defects and amino or organic acid disorders and screening for severe combined immunodeficiency syndrome.
Infants should routinely be positioned supine to minimize the risk of sudden infant death syndrome (SIDS). Prone positioning is contraindicated unless there are compelling clinical reasons for that position. Bed sharing with adults, tobacco exposure, overheating, soft items in the bed and prone positioning are associated with increased risk of SIDS.
FEEDING THE WELL NEONATE
A neonate is ready for feeding if he or she is (1) alert and vigorous, (2) has no abdominal distention, (3) has good bowel sounds, and (4) has a normal hunger cry. These signs usually occur within 6 hours after birth, but fetal distress or traumatic delivery may prolong this period. The healthy full-term infant should be allowed to feed every 2–5 hours on demand. The first breast feeding may occur in the delivery room. For formula-fed infants, the first feeding usually occurs by 3 hours of life. The feeding volume generally increases from 0.5 to 1 oz per feeding initially to 1.5–2 oz per feeding on day 3. By day 3, the average full-term newborn takes about 100 mL/kg/d of milk.
A wide range of infant formulas satisfy the nutritional needs of most neonates. Breast milk is the standard on which formulas are based (see Chapter 11). Despite low concentrations of several vitamins and minerals in breast milk, bio-availability is high. All the necessary nutrients, vitamins, minerals, and water are provided by human milk for the first 6 months of life except vitamin K (1 mg IM is administered at birth), vitamin D (400 IU/d for all infants beginning shortly after birth), and vitamin B12 and zinc (if the mother is a strict vegetarian and takes no supplements). Other advantages of breast milk include (1) immunologic, antimicrobial, and anti-inflammatory factors such as immunoglobulin A (IgA) and cellular, protein, and enzymatic components that decrease the incidence of upper respiratory and gastrointestinal (GI) infections; (2) possible decreased frequency and severity of childhood eczema and asthma; (3) improved mother-infant bonding; and (4) improved neurodevelopmental outcome.
Although about 70% of mothers in the United States start by breast feeding, only 33% continue to do so at 6 months. Hospital practices that facilitate successful initiation of breast feeding include rooming-in, nursing on demand, and avoiding unnecessary supplemental formula. Nursery staff must be trained to recognize problems associated with breast feeding and provide help and support for mothers in the hospital. An experienced professional should observe and assist with several feedings to document good latch-on. Good latch-on is important in preventing the common problems of sore nipples, unsatisfied infants, breast engorgement, poor milk supply, and hyperbilirubinemia.
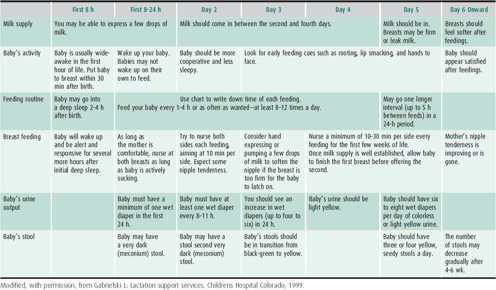
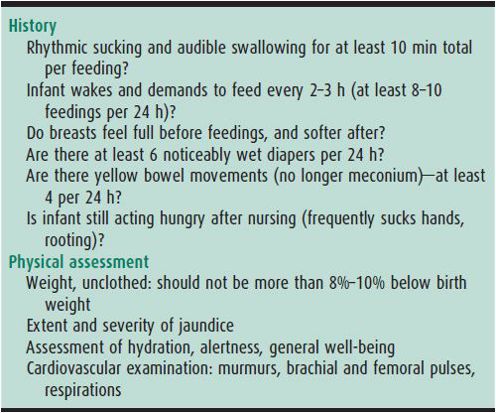
Table 2–4 presents guidelines the nursing mother and healthcare provider can use to assess successful breast feeding.
Table 2–4. Guidelines for successful breast feeding.
Abrams SA: Dietary guidelines for calcium and vitamin D: a new era. Pediatrics 2011;127:566 [PMID: 21339264].
American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome: SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics 2011;128:1030 [PMID: 22007004].
Lawrence RM, Lawrence RA: Breastfeeding: more than just good nutrition. Pediatr Rev 2011;32:267 [PMID: 21724901].
Martinez JA, Ballew MP: Infant formulas. Pediatr Rev 2011;32:179 [PMID: 21536776].
Moon RY, Fu L: Sudden infant death syndrome: an update. Pediatr Rev 2012;33:314 [PMID: 22855928].
Silvers KM et al: Breastfeeding protects against current asthma up to 6 years of age. J Pediatr 2012;160:991 [PMID: 22289356].
Smith EA et al: The national perinatal hepatitis B prevention program, 1994-2008. Pediatrics 2012;129:609 [PMID: 22451702].
Wagner CL, Greer FR: Section on breastfeeding and committee on nutrition: prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142 [PMID: 18977996].
Warren JB, Phillipi CA: Care of the well newborn. Pediatr Rev 2012;33:109 [PMID:222109929].
EARLY DISCHARGE OF THE NEWBORN INFANT
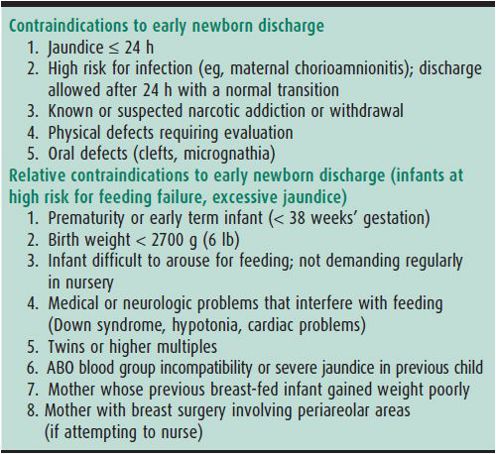
Discharge at 24–36 hours of age is safe and appropriate for some newborns if there are no contraindications (Table 2–5) and if a follow-up visit within 48 hours is ensured. Most infants with cardiac, respiratory, or infectious disorders are identified in the first 12–24 hours of life. The exception may be the infant treated intrapartum with antibiotic prophylaxis for maternal group B streptococcal (GBS) colonization or infection. The Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics (AAP) recommend that such infants be observed in hospital for 48 hours if they received no or inadequate intrapartum antibiotic prophylaxis (< 4 hours prior to delivery, or drug other than ampicillin, penicillin, or cefazolin). Hospital observation beyond 24 hours may not be necessary for well-appearing full-term infants who received adequate intrapartum chemoprophylaxis (penicillin, ampicillin, or cefazolin ≥ 4 hours prior to delivery), and for whom ready access to medical care can be ensured if needed. Other problems, such as jaundice and breast-feeding problems, typically occur after 48 hours and can usually be dealt with on an outpatient basis.
Table 2–5. Contraindications to early newborn discharge.
The AAP recommends a follow-up visit within 48 hours for all newborns discharged before 72 hours of age. Infants who are small or late preterm—especially if breast feeding—are at particular risk for inadequate intake; the early visit is especially important for these infants. Suggested guidelines for the follow-up interview and physical examination are presented in Table 2–6. The optimal timing of discharge must be determined in each case based on medical, social, and financial factors.
Table 2–6. Guidelines for early outpatient follow-up evaluation.
CIRCUMCISION
Circumcision is an elective procedure to be performed only in healthy, stable infants. The procedure has medical benefits, including prevention of phimosis, paraphimosis, balanoposthitis, and urinary tract infection. Important later benefits of circumcision include decreased incidence of penile cancer, decreased incidence of sexually transmitted diseases (including HIV), and decreased incidence of cervical cancer in female sexual partners. Most parental decisions regarding circumcision are religious and social, not medical. The risks of circumcision include local infection, bleeding, removal of too much skin, and urethral injury. The combined incidence of complications is less than 1%. Local anesthesia by dorsal penile nerve block or circumferential ring block using 1% lidocaine without epinephrine, or topical anesthetic cream are safe and effective methods that should always be used. Techniques allowing visualization of the glans throughout the procedure (Plastibell and Gomco clamp) are preferred to blind techniques (Mogen clamp) as occasional amputation of the glans occurs with the latter technique. Circumcision is contraindicated in infants with genital abnormalities (eg, hypospadias). A coagulation screen should be performed prior to the procedure in infants with a family history of serious bleeding disorders.
HEARING SCREENING
Normal hearing is critical to normal language development. Significant bilateral hearing loss is present in 1–3 infants per 1000 well neonates and in 2–4 per 100 neonates in the intensive care unit population. Infants should be screened for hearing loss by auditory brainstem evoked responses or evoked otoacoustic emissions as early as possible because up to 40% of hearing loss will be missed by risk analysis alone. Primary care providers and parents should be advised of the possibility of hearing loss and offered immediate referral in suspect cases. With the use of universal screening, the average age at which hearing loss is confirmed has dropped from 24–30 months to 2–3 months. If remediation is begun by 6 months, language and social development are commensurate with physical development.
American Academy of Pediatrics: Committee on the fetus and newborn: policy statement-hospital stay for healthy term newborns. Pediatrics 2010;125:405 [PMID: 20100744].
American Academy of Pediatrics Task Force on Circumcision: Circumcision policy statement. Pediatrics 2012;130:585 [PMID: 22926180].
Berg AL et al: Hearing screening in a well infant nursery: profile of automated ABR-fail/OAE-pass. Pediatrics 2011;127:269 [PMID: 21262886].
Prevention of perinatal Group B Streptococcal disease revised guidelines from CDC, 2010. MMWR 2010;59:1–32 [PMID: 21088663].
US Preventive Services Task Force: Universal screening for hearingloss in newborns: US Preventive Services Task Force recommendation statement. Pediatrics 2008;122:143 [PMID: 18595997].
COMMON PROBLEMS IN THE TERM NEWBORN
NEONATAL JAUNDICE
 General Considerations
General Considerations
Sixty-five percent of newborns develop visible jaundice with a total serum bilirubin (TSB) level higher than 6 mg/dL during the first week of life. Bilirubin, a potent antioxidant and peroxyl scavenger, may protect the normal newborn, who is deficient in antioxidants such as vitamin E, catalase, and super-oxide dismutase, from oxygen toxicity in the first days of life. Approximately 8%–10% of newborns develop excessive hyperbilirubinemia (TSB > 17 mg/dL), and 1%–2% have TSB above 20 mg/dL. Extremely high and potentially dangerous TSB levels are rare. Approximately 1 in 700 infants have TSB higher than 25 mg/dL, and 1 in 10,000 have TSB above 30 mg/dL. Such high levels can cause kernicterus, characterized by injury to the basal ganglia and brainstem.
Kernicterus caused by hyperbilirubinemia was common in neonates with Rh-isoimmunization until the institution of exchange transfusion for affected infants and postpartum high-titer Rho (D) immune globulin treatment to prevent sensitization of Rh-negative mothers. For several decades after the introduction of exchange transfusion and phototherapy aimed at keeping the neonate’s TSB below 20 mg/dL, there were no reported cases of kernicterus in the United States. Since the early 1990s, however, there has been a reappearance of kernicterus, with more than 120 cases reported. Common factors in the recent cases are newborn discharge before 48 hours, breast feeding, delayed measurement of TSB, unrecognized hemolysis, lack of early post discharge follow-up, and failure to recognize the early symptoms of bilirubin encephalopathy.
Bilirubin is produced by the breakdown of heme (iron protoporphyrin) in the reticuloendothelial system and bone marrow. Heme is cleaved by heme oxygenase to iron, which is conserved; carbon monoxide, which is exhaled; and biliverdin, which is converted to bilirubin by bilirubin reductase. Each gram of hemoglobin yields 34 mg of bilirubin (1 mg/dL = 17.2 mmol/L of bilirubin). This unconjugated bilirubin is bound to albumin and carried to the liver, where it is taken up by hepatocytes. In the presence of the enzyme uridyldiphosphoglucuronyl transferase (UDPGT; glucuronyl transferase), bilirubin is conjugated to one or two glucuronide molecules. Conjugated bilirubin is then excreted through the bile to the intestine. In the presence of normal gut flora, conjugated bilirubin is metabolized to stercobilins and excreted in the stool. Absence of gut flora and slow GI motility, both characteristics of the newborn, cause stasis of conjugated bilirubin in the intestinal lumen, where mucosal β-glucuronidase removes the glucuronide molecules and leaves unconjugated bilirubin to be reabsorbed (enterohepatic circulation).
Excess accumulation of bilirubin in blood depends on both the rate of bilirubin production and the rate of excretion. It is best determined by reference to an hour-specific TSB level above the 95th percentile for age in hours (Figure 2–2).
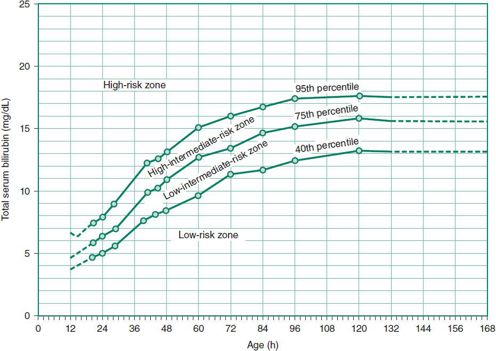
 Figure 2–2. Risk designation of full-term and near-term newborns based on their hour-specific bilirubin values. (Reproduced, with permission, from Bhutani VK et al: Predictive ability of a predischarge hour-specific serum bilirubin test for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103:6.)
Figure 2–2. Risk designation of full-term and near-term newborns based on their hour-specific bilirubin values. (Reproduced, with permission, from Bhutani VK et al: Predictive ability of a predischarge hour-specific serum bilirubin test for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103:6.)
1. Physiologic Jaundice
Factors contributing to physiologic jaundice in neonates include low UDPGT activity, relatively high red cell mass, absence of intestinal flora, slow intestinal motility, and increased enterohepatic circulation of bilirubin in the first days of life. Hyperbilirubinemia outside of the ranges noted in Figure 2–2 is not physiologic and requires further evaluation.
2. Pathologic Unconjugated Hyperbilirubinemia
Pathologic unconjugated hyperbilirubinemia can be grouped into two main categories: overproduction of bilirubin or decreased conjugation of bilirubin (Table 2–7). The TSB is a reflection of the balance between these processes. Visible jaundice with a TSB greater than 5 mg/dL before 24 hours of age is most commonly a result of significant hemolysis.
Table 2–7. Causes of pathologic unconjugated hyperbilirubinemia.

A. Increased Bilirubin Production
Increased bilirubin production is caused by excessive destruction of neonatal red blood cells. Destruction may be mediated by maternal antibodies (Coombs test–positive), or may be due to abnormal red cell membranes (spherocytosis), or abnormal red cell enzymes (glucose-6-phosphate dehydrogenase [G6PD] deficiency) causing decreased red cell life span not mediated by antibodies. Antibodies can be directed against the major blood group antigens (type A or type B infant of a type O mother); the antigens of the Rh-system (D, E, C, d, e, c); and Kell, Duffy, and other antigens.
1. Antibody-mediated hemolysis (Coombs test–positive)
A. ABO BLOOD GROUP INCOMPATIBILITY—This finding can accompany any pregnancy in a type O mother. Hemolysis is usually mild, but the severity is unpredictable because of variability in the amount of naturally occurring maternal anti-A or anti-B IgG antibodies. Although 20% of pregnancies are “set-ups” for ABO incompatibility (mother O, infant A or B), only 33% of infants in such cases have a positive direct Coombs test and only 20% of these develop jaundice that requires therapy. Since maternal antibodies may persist for several months after birth, the newborn may become progressively more anemic over the first few weeks of life, occasionally to the point of requiring transfusion.
B. RH-ISOIMMUNIZATION—This hemolytic process is less common, more severe, and more predictable than ABO incompatibility. The severity increases with each immunized pregnancy because of an anamnestic maternal IgG antibody response. Most Rh-disease can be prevented by giving high-titer Rho (D) immune globulin to the Rh-negative woman after invasive procedures during pregnancy or after miscarriage, abortion, or delivery of an Rh-positive infant. Affected neonates are often anemic at birth, and continued hemolysis rapidly causes hyperbilirubinemia and more severe anemia. The most severe form of Rh-isoimmunization, erythroblastosis fetalis, is characterized by life-threatening anemia, generalized edema, and fetal or neonatal heart failure. Without antenatal intervention, fetal or neonatal death often results. The cornerstone of antenatal management is transfusion of the fetus with Rh-negative cells, either directly into the umbilical vein or into the fetal abdominal cavity. Phototherapy is usually started in these infants upon delivery, with exchange transfusion frequently needed. Intravenous immune globulin (IVIG; 0.5–1 g/kg) given to the infant as soon as the diagnosis is made may decrease the need for exchange transfusion. Ongoing hemolysis occurs until all maternal antibodies are gone; therefore, these infants require monitoring for 2–3 months for recurrent anemia severe enough to require transfusion.
2. Nonimmune hemolysis (Coombs test–negative)
A. HEREDITARY SPHEROCYTOSIS—This condition is the most common of the red cell membrane defects and causes hemolysis by decreasing red cell deformability. Affected infants may have hyperbilirubinemia severe enough to require exchange transfusion. Splenomegaly may be present. Diagnosis is suspected by peripheral blood smear and family history. See Chapter 30 for a more in-depth discussion.
B. G6PD DEFICIENCY—This condition is the most common red cell enzyme defect causing hemolysis, especially in infants of African, Mediterranean, or Asian descent. Onset of jaundice is often later than in isoimmune hemolytic disease, toward 1 week of age. The role of G6PD deficiency in neonatal jaundice is probably underestimated as up to 10%–13% of African Americans are G6PD-deficient. Although the disorder is X-linked, female heterozygotes are also at increased risk of hyperbilirubinemia due to X-chromosome inactivation. In most cases, no triggering agent for hemolysis is found in the newborn. Rather, some infants who develop severe jaundice with G6PD deficiency have been found also to have Gilbert syndrome (see below). Their increased bilirubin production is further exaggerated by a decreased rate of bilirubin conjugation. Since G6PD enzyme activity is high in reticulocytes, neonates with a large number of reticulocytes may have falsely normal enzyme tests. A low G6PD level should always raise suspicions. Repeat testing in suspect cases with initially normal results is indicated at 2–3 months of age. Please also see Chapter 30 for more details.
3. Nonhemolytic increased bilirubin production—Enclosed hemorrhage, such as cephalohematoma, intracranial hemorrhage, or extensive bruising in the skin, can lead to jaundice. Polycythemia leads to jaundice by increased red cell mass, with increased numbers of cells reaching senescence daily. Bowel obstruction, functional or mechanical, leads to an increased enterohepatic circulation of bilirubin.
B. Decreased Rate of Conjugation
1. UDPGT deficiency: Crigler-Najjar syndrome type I (complete deficiency, autosomal recessive) and type II (partial deficiency, autosomal dominant)—These rare conditions result from mutations in the exon or encoding region of the UDPGT gene that cause complete or nearly complete absence of enzyme activity. Both can cause severe unconjugated hyperbilirubinemia, bilirubin encephalopathy, and death if untreated. In type II, the enzyme can be induced with phenobarbital, which may lower bilirubin levels by 30%–80%. Liver transplantation is curative.
2. Gilbert syndrome—This is a common mild autosomal dominant disorder characterized by decreased hepatic UDPGT activity caused by genetic polymorphism at the promoter region of the UDPGT gene. Approximately 9% of the population is homozygous, and 42% is heterozygous for this abnormality, with a gene frequency of 0.3. Affected individuals tend to develop hyperbilirubinemia in the presence of conditions that increase bilirubin load, including G6PD deficiency. They are also more likely to have prolonged neonatal jaundice and breast-milk jaundice.
C. Hyperbilirubinemia Caused by Unknown or Multiple Factors
1. Racial differences—Asians (23%) are more likely than whites (10%–13%) or African Americans (4%) to have a peak neonatal TSB greater than 12 mg/dL (206 mmol/L). It is likely that these differences result from racial variations in prevalence of UDPGT gene polymorphisms or associated G6PD deficiency.
2. Prematurity—Premature infants often have poor enteral intake, delayed stooling, and increased enterohepatic circulation, as well as a shorter red cell life. Infants at 35–36 weeks’ gestation are 13 times more likely than term infants to be readmitted for hyperbilirubinemia. Even early-term infants (37–38 weeks’ gestation) are four times more likely than term neonates to have TSB greater than 13 mg/dL (224 mmol/L).
3. Breast feeding and jaundice
A. BREAST-MILK JAUNDICE—Unconjugated hyperbilirubinemia lasting until 2–3 months of age is common in breast-fed infants. An increased prevalence of the Gilbert syndrome promoter polymorphism may be involved. Moderate unconjugated hyperbilirubinemia for 6–12 weeks in a thriving breast-fed infant without evidence of hemolysis, hypothyroidism, or other disease strongly suggests this diagnosis.
B. BREAST FEEDING–ASSOCIATED JAUNDICE—This common condition has also been called “lack-of-breast-milk” jaundice. Breast-fed infants have a higher incidence (9%) of unconjugated serum bilirubin levels greater than 13 mg/dL (224 mmol/L) than do formula-fed infants (2%) and are more likely to have TSB greater than 15 mg/dL (258 mmol/L) than formula-fed infants (2% vs 0.3%). The pathogenesis is probably poor enteral intake and increased enterohepatic circulation. There is no apparent increase in bilirubin production as measured by carbon monoxide exhalation. Although rarely severe enough to cause bilirubin encephalopathy, nearly 100% of the infants with kernicterus reported over the past 20 years were exclusively breast fed, and in 50%, breast feeding was the only known risk factor. Excessive jaundice should be considered a possible sign of failure to establish an adequate milk supply, and should prompt specific inquiries (Table 2–8). If intake is inadequate, the infant should receive supplemental formula and the mother should be instructed to nurse more frequently and to use an electric breast pump every 2 hours to enhance milk production. Consultation with a lactation specialist should be considered. Because hospital discharge of normal newborns occurs before the milk supply is established and before jaundice peaks, a follow-up visit 2 days after discharge is recommended by the AAP to evaluate adequacy of intake and degree of jaundice.
Table 2–8. Signs of inadequate breast-milk intake.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







