Background
Despite intensive efforts directed at initial training in fetal heart rate interpretation, continuing medical education, board certification/recertification, team training, and the development of specific protocols for the management of abnormal fetal heart rate patterns, the goals of consistently preventing hypoxia-induced fetal metabolic acidemia and neurologic injury remain elusive.
Objective
The purpose of this study was to validate a recently published algorithm for the management of category II fetal heart rate tracings, to examine reasons for the birth of infants with significant metabolic acidemia despite the use of electronic fetal heart rate monitoring, and to examine critically the limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia.
Study Design
The potential performance of electronic fetal heart rate monitoring under ideal circumstances was evaluated in an outcomes-blinded examination fetal heart rate tracing of infants with metabolic acidemia at birth (base deficit, >12) and matched control infants (base deficit, <8) under the following conditions: (1) expert primary interpretation, (2) use of a published algorithm that was developed and endorsed by a large group of national experts, (3) assumption of a 30-minute period of evaluation for noncritical category II fetal heart rate tracings, followed by delivery within 30 minutes, (4) evaluation without the need to provide patient care simultaneously, and (5) comparison of results under these circumstances with those achieved in actual clinical practice.
Results
During the study period, 120 infants were identified with an arterial cord blood base deficit of >12 mM/L. Matched control infants were not demographically different from subjects. In actual practice, operative intervention on the basis of an abnormal fetal heart rate tracings occurred in 36 of 120 fetuses (30.0%) with metabolic acidemia. Based on expert, algorithm-assisted reviews, 55 of 120 patients with acidemia (45.8%) were judged to need operative intervention for abnormal fetal heart rate tracings. This difference was significant ( P =.016). In infants who were born with a base deficit of >12 mM/L in which blinded, algorithm-assisted expert review indicated the need for operative delivery, the decision for delivery would have been made an average of 131 minutes before the actual delivery. The rate of expert intervention for fetal heart rate concerns in the nonacidemic control group (22/120; 18.3%) was similar to the actual intervention rate (23/120; 19.2%; P =1.0) Expert review did not mandate earlier delivery in 65 of 120 patients with metabolic acidemia. The primary features of these 65 cases included the occurrence of sentinel events with prolonged deceleration just before delivery, the rapid deterioration of nonemergent category II fetal heart rate tracings before realistic time frames for recognition and intervention, and the failure of recognized fetal heart rate patterns such as variability to identify metabolic acidemia.
Conclusions
Expert, algorithm-assisted fetal heart rate interpretation has the potential to improve standard clinical performance by facilitating significantly earlier recognition of some tracings that are associated with metabolic acidemia without increasing the rate of operative intervention. However, this improvement is modest. Of infants who are born with metabolic acidemia, only approximately one-half potentially could be identified and have delivery expedited even under ideal circumstances, which are probably not realistic in current US practice. This represents the limits of electronic fetal heart rate monitoring performance. Additional technologies will be necessary if the goal of the prevention of neonatal metabolic acidemia is to be realized.
The impact of electronic fetal heart rate monitoring (EFHRM) on neonatal outcomes continues to be controversial. Although unexpected intrapartum fetal death has been largely eliminated with such monitoring, the goal of consistently preventing hypoxia-induced fetal metabolic acidemia, a condition with a well-defined relationship to neonatal encephalopathy, remains elusive. Even less well-documented is the impact of EFHRM on long-term neonatal outcomes; because conditions such as cerebral palsy are multifactorial in origin, any potential impact of monitoring on such outcomes is so small as to never have been statistically demonstrated, despite intensive efforts directed at initial training in fetal heart rate interpretation, continuing medical education, board certification/recertification, team training, and the development of specific protocols for the management of abnormal fetal heart rate patterns. We sought to examine reasons for the birth of infants with significant metabolic acidemia despite the use of EFHRM, to assess the potential impact of a previously published algorithm on these outcomes, and to examine critically the limits of EFHRM in the prevention of neonatal metabolic acidemia.
Materials and Methods
This study met criteria set forth in [45 CFR 46.101 (b), Category [4]] and was given exempt status by the Institutional Review Board of the MedStar Health Research Institute. We performed a case control study that compared 2 groups, a study group with fetal metabolic academia at birth and a group with normal cord gases.
Our study groups were drawn from all women who delivered from January 1, 2012, to December 31, 2013, in 2 academically affiliated community teaching hospitals in the Baltimore-Washington corridor. One facility practiced universal umbilical cord gas analysis; the second facility encouraged the liberal use of such analysis but did not insist on it in all births. Inclusion criteria were singleton, vertex-presenting fetuses at ≥37 weeks gestation with EFHRM and umbilical artery blood gas measurements. Women with ≥1 previous cesarean deliveries were excluded. Fetal heart rate tracings (FHRTs) were archived electronically along with other data from the labor and delivery record with the use of the PeriCALM Tracings and PeriBirth data collection systems (PeriGen, Cranbury, NJ). Women who delivered infants with arterial cord blood metabolic acidemia (base deficit [BD], >12 mM/L) formed the index study group. Each index case was then inserted randomly back into a temporally ordered database of all patients, and the nearest patient who was matched for multiparous or nulliparous status who delivered an infant with BD < 8 mM/L was selected as a control.
For patients and controls, each FHRT was examined in its entirety by 1 of 3 authors (S.L.C., T.J.G., and A.T.) who applied to it a previously published algorithm for the management of category II FHRTs to determine when, if ever, the algorithm would have called for intervention ( Figure 1 ). The reviewers were blinded to cord gas values and whether any patient was included as a case or a control. To accomplish this blinding, 1 author (E.F.H.) organized and distributed the randomly combined set of normal and acidemic tracings but did not participate in the primary FHRT reviews.
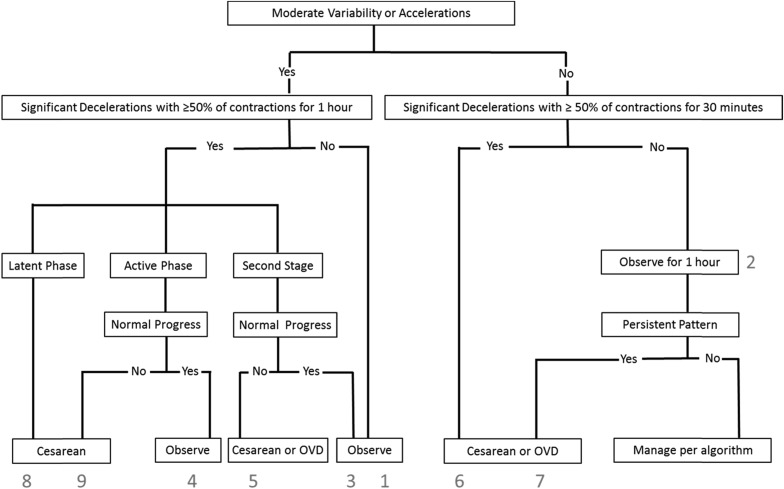
After these blinded reviews of individual cases, a secondary review was performed by all reviewers for babies who were born with metabolic acidemia in which the initial reviewer did not recommend intervention (65 cases) based either on their clinical judgment or the use of the algorithm. Data regarding actual timing and the route of delivery, the indication for operative delivery, and newborn outcomes were also evaluated.
We then asked the following questions:
- 1.
How did delivery decisions based on this algorithm-assisted review differ from those made in actual clinical practice? (A difference in cesarean rates for fetal indications in fetuses with and without metabolic acidemia between study and control groups would allow us to address this issue.)
- 2.
What was the nature of the FHRTs in infants born with metabolic acidemia that were not predicted or potentially prevented even with FHRT interpretation by these experts? (This issue would be addressed by analysis of these individual tracings to identify common features of those fetuses who were born with metabolic acidemia despite monitoring.)
- 3.
What are the limits of the ability of FHR monitoring to predict and prevent the occurrence of metabolic acidemia during labor? (This issue would be addressed by examination of the performance of an algorithm developed by a group of recognized experts and the structuring of the study to account for variables other than the intrinsic ability of FHR monitoring to demonstrate recognized abnormal FHR patterns.)
Proportions were compared using chi-squared or Fisher’s exact test or the Mann-Whitney test for nonnormal distributions, as appropriate. Normally distributed variables were compared with the use of the Student t test. All tests were 2-tailed, and a probability value of <.05 was considered to be significant.
Results
During the study period, 120 infants were identified with arterial cord blood BD > 12 mM/L, which represents 1.2% of the 9888 women who met the potential enrollment criteria and who delivered during this time frame and 2.2% of the 5510 infants with arterial cord blood gas sampling. Demographic characteristics for the study and control groups were similar ( Table 1 ). The facilities that performed elective vs uniform blood gas analysis accounted for 9 and 111 of these cases, respectively.
| Variable | Group | P value | |
|---|---|---|---|
| Control (n=120) | Metabolic acidosis (n=120) | ||
| Maternal age, median (interquartile range) | 25.5 (20–30) | 27 (21–32) | .11 |
| Birthweight, median (interquartile range) | 3293 (2989–3662) | 3369 (3111–3686) | .46 |
| Gestational age, median (interquartile range) | 39.9 (39.0–40.5) | 39.9 (39.1–40.6) | .89 |
| Body mass index, median (interquartile range) | 31.2 (27.7–35.4) | 30.8 (27.6–37.1) | .63 |
| Nulliparity, n (%) | 96 (80.0) | 96 (80.0) | 1 |
| Diabetes mellitus, n (%) | 5 (4.2) | 7 (5.8) | .76 |
| Hypertension, n (%) | 14 (11.7) | 21 (17.5) | .27 |
| Epidural, n (%) | 60 (50.0) | 40 (33.3) | .013 |
The distribution of cases according to both cord arterial pH and BD are presented in Table 2 . The median times from end of FHRT to delivery were 1.8 and 1.4 minutes for cases and control subjects, respectively. The 25th and 75th percentiles for these 2 groups were 0.4/0.5 and 9.2/8.4 minutes.
| pH | Base deficit, n (%) | ||
|---|---|---|---|
| <8 mM/L (n=120) | 12–15 mM/L (n=94) | >15 mM/L (n=26) | |
| <7.10 (n=83) | 0 | 58 (24.2) | 25 (10.4) |
| 7.10–7.15 (n=32) | 11 (4.6) | 20 (8.3) | 1 (0.4) |
| >7.15 (n=125) | 109 (45.4) | 16 (6.7) | 0 |
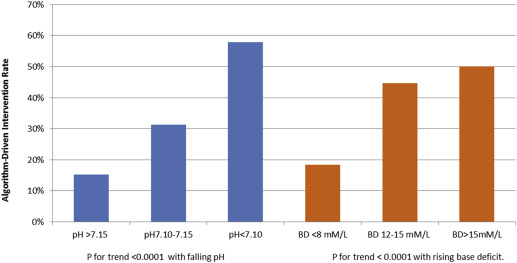
In actual practice, operative intervention on the basis of an abnormal FHRT occurred in 36 of 120 fetuses (30.0%) with metabolic acidemia. Based on the expert, algorithm- assisted reviews, 55 of these 120 patients with acidemia (45.8%) were judged as needing operative intervention for abnormal FHRT. This difference was significant ( P =.016). In infants born who were with BD >12 mM/L for whom the blinded, algorithm-assisted expert review indicated the need for operative delivery based on an abnormal FHRT, the decision for delivery would have been made an average of 131 minutes before the actual delivery. Among the 24 patients with metabolic acidemia who ultimately did deliver by cesarean section for an abnormal FHRT, such intervention was judged necessary by expert review in 20 patients and, on average, 80 minutes before actual birth. In the 68 patients with metabolic acidemia who delivered vaginally, earlier intervention was indicated, per expert review in 19 patients and, on average, 178 minutes before actual birth. The rate of expert intervention for FHR concerns in the nonacidemic control group (22/120; 18.3%) was similar to the actual intervention rate (23/120; 19.2%; P =1.0) Figure 2 shows the algorithm-assisted expert intervention rates according to both cord arterial pH and BD. There was a highly significant trend ( P =.0001) toward greater rates of intervention with both falling pH and rising BD. Table 3 presents the relationship between BD and adverse newborn infant outcome. The trend toward increased rates of several adverse outcomes with rising BD was highly significant. However, even with BD values exceeding 15 mM/L, adverse outcomes were present in only a few infants.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


