Materials and Methods
Criteria for study selection
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. All published cohort studies and randomized trials (including at least 5 patients) that investigated the impact of excision of benign nonendometriotic ovarian cysts on ovarian reserve as determined by changes in postoperative serum anti-Müllerian hormone concentration were included in this metaanalysis.
Outcome measures
Primary measure
This included postoperative changes in serum anti-Müllerian hormone concentration.
Secondary measures
These included postoperative changes in serum follicle-stimulating hormone concentration and antral follicle count.
Search strategy
An extensive electronic database search was performed using MEDLINE, Scopus, Embase, and ScienceDirect to identify published research articles between January 2000 and Jan. 31, 2016, on the impact of excision of benign ovarian cysts (excluding endometriomas) on ovarian reserve as determined by serum anti-Müllerian hormone concentration. No restrictions were placed on language. A combination of the following search terms was used: laparoscopy, laparotomy, ovarian cystectomy, excision, anti-Müllerian hormone, benign ovarian cysts, and ovarian reserve. All searches were carried out by the first author and then independently repeated using the same criteria by an accredited clinical librarian. All relevant reports were retrieved, and their reference lists were reviewed manually to identify further studies. A manual search of related articles on PubMed was also performed. We also considered published abstracts from conferences.
Data extraction
All the identified papers were evaluated according to a standardized format including study design, methods, participant characteristics, intervention, and results. Two investigators scored the studies and collected the information independently. In the case of discrepancies in scoring between the 2 investigators, a consensus was reached after discussion or after involvement of the senior investigator. In 5 studies the mean ± SD was not presented. The authors of these studies were contacted, but only 2 replied, providing the missing data, which were used in our analysis.
In another study, which was a conference abstract, the authors did not describe the methods of recruitment (inclusion and exclusion criteria), nor they specified the type of anti-Müllerian hormone assay kit. This study was included in the initial analysis but was excluded from the sensitivity analysis. The authors were contacted to provide the missing information, but no response was received.
Quality of included studies and risk of bias assessment
The quality and risk of bias of the included studies were assessed using the modified Newcastle-Ottawa Scale, as previously described. The original Newcastle-Ottawa Scale assesses 3 main categories including selection, comparability, and outcomes, giving a maximum of 4, 2, and 3 stars for each category, respectively. This scale was modified to suit the nature of this study, giving a maximum of 3 stars for selection, 4 for comparability, and 2 for outcome criteria.
Selection was rated according to recruitment bias, selection of consecutive patients, and power calculation. Comparability was assessed based on studies adjusting their analysis for 4 confounders including patients’ age (<40 years), cyst diameter (>5 cm), baseline serum anti-Müllerian hormone (≥ 3.1 ng/mL), and cyst laterality.
Outcome was scored according to completeness of at least 3 months of follow-up after surgery. It is generally agreed that a limit of 5 stars could identify studies at low risk of bias. However, in this study, we have given more weight to comparability factors and used the cutoff level of 6 stars, with a minimum of 3 stars in the comparability category. Table 1 shows the results of quality scores of the studies included in this analysis.
| Author | Year | Selection | Comparability | Outcome | Total score |
|---|---|---|---|---|---|
| Chang et al | 2010 | * | * | ** | 4 |
| Iwase et al | 2010 | ** | *** | * | 6 |
| Mohamed et al | 2011 | ** | **** | ** | 8 |
| Kim et al | 2013 | * | * | ** | 4 |
| Chen et al | 2014 | ** | *** | * | 6 |
| Huang et al | 2014 | ** | **** | * | 7 |
| Kwon et al | 2014 | ** | **** | ** | 8 |
| Yoon et al | 2014 | ** | *** | ** | 7 |
| Amooee et al | 2015 | ** | *** | ** | 7 |
| Ergun et al | 2015 | * | **** | ** | 7 |
Data analysis
Pre- and postoperative data including mean ± SD serum concentrations of anti-Müllerian hormone (nanograms per milliliter) and follicle-stimulating hormone (milliinternational units per milliliter) and antral follicle count were extracted from the individual studies and pooled using RevMan software (Review Manager, version 5.1, The Cochrane Collaboration, 2011; The Nordic Cochrane Centre, Copenhagen, Denmark).
The weighted mean difference between the pre- and postoperative values was calculated. Statistical heterogeneity was assessed by a χ 2 test and I 2 statistics. A χ 2 statistic larger than its degree of freedom or an I 2 higher than 50% was indicative of significant heterogeneity between studies. When heterogeneity was significant, a random-effect model was used for metaanalysis. A fixed effect metaanalysis was used when there was no significant heterogeneity.
The initial analysis included data from all studies, irrespective of length of follow-up and cyst characteristics (diameter, laterality, and pathological type). In studies with multiple postoperative measurements at different follow-up points, we used the latest anti-Müllerian hormone level. Further subgroup analyses of anti-Müllerian hormone levels were then performed based on the laterality of the excised cysts, anti-Müllerian hormone kits used, and duration of follow-up. To examine and account for heterogeneity, a sensitivity analysis was carried out based on modified the Newcastle-Ottawa Scale for risk of bias as described above. Studies with the lowest risk of bias were defined as those with a score of ≥6 with at least 3 stars on comparability score and using the same surgical approach (laparoscopy).
Results
A total of 25 articles were identified ( Figure 1 ). Initially all articles were screened on the basis of the title and abstract to exclude studies that were not relevant to our objectives. As a result, 10 articles were viewed in full.
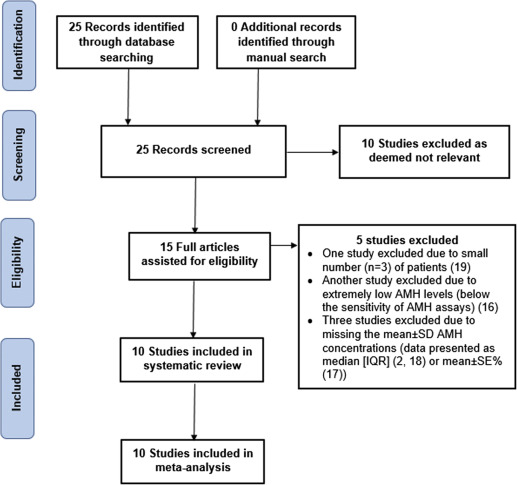
Excluded studies
After the initial screening on the basis of the title and abstract, 10 studies did not use the anti-Müllerian hormone to investigate ovarian reserve after surgery for benign nonendometriotic cyst and were therefore excluded. Five further studies were excluded, one because of the small number of patients (n = 3), another study because of extremely low anti-Müllerian hormone levels (below the sensitivity of anti-Müllerian hormone assays), and 3 studies because of missing the mean ± SD of serum anti-Müllerian hormone concentrations (data were either presented as median [interquartile range]) or mean ± SE percentage. The authors of the latter 3 studies were contacted to provide the anti-Müllerian hormone data, but no response was received.
Included studies
Details of the included 10 studies are shown in Table 2 .
| Author | Country | Design | n | Age, mean ± SD | Laterality | Cyst diameter, mean ± SD | FU, mo | Primary outcome | Secondary Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Chang et al, 2010 | Korea | Prospective cohort | 7 | 33.75 ± 7.20 | Not specified | Not specified | 3 | AMH (DSL kit), | — |
| Iwase et al, 2011 | Japan | Prospective cohort | 21 | 29.40 ± 7.3 | Uni = 16 Bil = 5 | Not specified | 1 | AMH (IOT kit), | FSH |
| Mohamed et al, 2011 | Egypt | RCT a | Arm 1 = 30 Arm 2 = 29 | 23.00 ± 4.1 | All unilateral | Arm 1, 5.1 ± 2.2 Arm 2, 5.6 ± 2.0 | 6 | AMH (DSL kit), | AFC |
| Kim et al, 2013 | Korea | Prospective cohort | 34 | Not specified | Not specified | Not specified | 3 | AMH (kit not specified) | — |
| Chen et al, 2014 | China | Prospective cohort | 22 | 29.95 ± 3.92 | Uni = 18 Bil = 4 | 6.35 ± 2.88 | 1 | AMH (Ansh Labs) | — |
| Huang et al, 2014 | Taiwan | Retrospective case control | 71 | 34.59 ± 10.18 | Uni = 67 Bil = 4 | 7.05 ± 2.37 | 1 | AMH (DSL kit), | FSH |
| Kwon et al, 2014 | Korea | Prospective cohort | 32 | 30.00 ± 6.23 | Uni = 24 Bil = 8 | 7.28 ± 2.80 | 3 | AMH (original Gen II) | — |
| Yoon et al, 2014 | Korea | Prospective cohort | 37 | 30.30 ± 5.0 | Not specified | 7.28 ± 2.80 | 3 | AMH (IOT kit) | — |
| Amooee et al, 2015 | Iran | Prospective cohort | 60 | 25.80, average b | Not specified | 7.6, average b | 3 | AMH (DSL kit) | — |
| Ergun et al, 2015 | Turkey | Prospective cohort | 24 | 28.39 ± 6.76 | Uni = 22 Bil = 2 | 5.9 ± 1.98 | 3 | AMH (modified Gen II) | FSH |
a RCT, arm 1, laparoscopy; arm 2, laparotomy
Study design
The included studies were all cohort studies except one, which was a randomized controlled trial. However, both arms of the randomized controlled trial (laparoscopy and laparotomy) were combined and included in the initial metaanalysis as a cohort study. One arm (laparoscopy) was then included in the sensitivity analysis.
Participants
Selection criteria were appropriate for all studies. All participants underwent the same type of surgery (cystectomy) through laparoscopy except one study, in which the patients were randomly allocated to either laparoscopy or laparotomy. Patients of this randomized controlled trial were consecutive and were followed up within their particular group, and the results were given separately for each arm of the randomized controlled trial. All studies reported inclusion and exclusion criteria that were appropriate except one. The author of that study was contacted but did not reply. All patients were accounted for in all studies.
Ovarian cyst diagnosis
Most of the studies reported that the initial diagnosis of the cysts was achieved through transvaginal ultrasound. The ultrasound scans were performed by gynecologists with sufficient experience. Postoperatively, the nature of the cyst was confirmed with histopathological examination. Six studies reported the mean ± SD cyst diameter and 6 studies determined the side and laterality of the cysts.
Surgery and length of follow-up
All studies included patients undergoing ovarian cystectomy carried out laparoscopically except 1 study, which was an randomized controlled trial comparing laparoscopic vs open cystectomy. The length of follow-up was 1 month in 4 studies, 3 months in 6 studies, and 6 months in 1 study.
Anti-Müllerian hormone kits
Anti-Müllerian hormone concentration was measured using 1 of the 4 available kits. The first one was Immunotech anti-Müllerian hormone/Müllerian-inhibiting substance enzyme immunoassay kit (Immunotech, Beckman Coulter, Marseille, France). The intra- and interassay coefficients of variation for the anti-Müllerian hormone assay were below 12.3% and 14.2%, respectively, with a detection limit of 0.14 ng/mL.
The second anti-Müllerian hormone kit was Diagnostic Systems Laboratories active Müllerian–inhibiting substance/anti-Müllerian hormone enzyme-linked immunosorbent assay kit (Diagnostic Systems Laboratories, Webster, TX). The intra- and interassay coefficients of variation for the anti-Müllerian hormone were 4.6% and 8.0%, respectively, with a detection limit of 0.017 ng/mL.
The third kit was the AMH Gen II enzyme-linked immunosorbent assay (Beckman Coulter, Chaska, MN). The intra- and interassay coefficients of variation for the anti-Müllerian hormone assay were both below 10%, with a detection limit of 0.08 ng/mL. Two studies used this kit including Ergun et al, who used the modified AMH Gen II kit and Kwon et al, who used the original Gen II assay. The fourth kit was the ultrasensitive anti-Müllerian hormone enzyme-linked immunosorbent assay Ansh Labs assay (Ansh Labs, Glasgow, United Kingdom). The intra- and interassay coefficients of variation for the anti-Müllerian hormone were 0.02 (2.22/95) and 7.81 (15.62/2), respectively, with a detection limit of 0.06 ng/mL.
Potential source of bias
In all studies, patients were selected in a consecutive fashion. The selection method was clearly described, making it easy to assess selection bias.
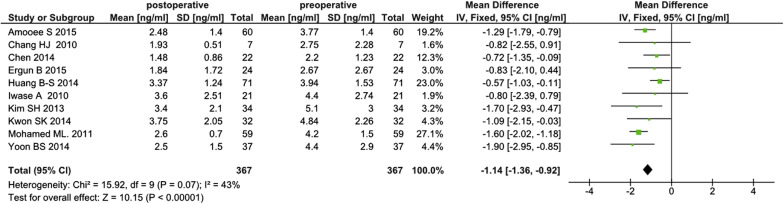
Overall pooled results for all studies
The initial analysis of the 10 studies included all 367 patients who underwent cystectomy for unilateral or bilateral benign nonendometriotic ovarian cysts. The analysis revealed a statistically significant postoperative fall in serum anti-Müllerian hormone concentrations (weighted mean difference, –1.14 ng/ml; 95% confidence interval, –1.36 to –0.92). Heterogeneity between studies was low (I 2 = 43 %) ( Figure 2 ).
Subgroup analysis
Laterality of benign nonendometriotic ovarian cysts
Six studies included 206 patients undergoing unilateral ovarian cystectomy. Results showed a statistically significant decline in serum anti-Müllerian hormone level after surgery (weighted mean difference, –0.97 ng/mL; 95% confidence interval, –1.58 to –0.37; I 2 = 73%). Bilateral ovarian cystectomy was reported in 5 studies including 23 patients. Pooled analysis of the data revealed no statistical significant change in postoperative serum anti-Müllerian hormone level (weighted mean difference, –0.80; 95% confidence interval, –1.76 to 0.16; I 2 = 0%).
Studies with different length of follow-up
Six studies (n = 270) with 1 month follow up revealed a statistically significant decline in serum anti-Müllerian hormone level (weighted mean difference, –1.16; 95% confidence interval, –1.69 to –0.63; I 2 = 76%). Similarly, 7 studies (n = 253) with a 3 month follow-up showed a statistically significant fall of serum anti-Müllerian hormone concentration after surgery (weighted mean difference, –1.44; 95% confidence interval, –1.71 to –1.16; I 2 = 0%).
Studies using different anti-Müllerian hormone assays
Analysis of 4 studies (n = 197) using the Diagnostic Systems Laboratories anti-Müllerian hormone kit revealed a statistically significant decline in postoperative anti-Müllerian hormone level (weighted mean difference, –1.18; 95% confidence interval, –1.85 to –0.52; I 2 = 81%). Pooled analysis of 2 studies (n = 56) using the Gen II anti-Müllerian hormone assay showed a statistically significant fall in postoperative serum anti-Müllerian hormone level (weighted mean difference, -0.88; 95% confidence interval, –1.71 to –0.04; I 2 = 0%). Two other studies (n = 58) using an IOT anti-Müllerian hormone assay revealed a statistically significant decline in postoperative anti-Müllerian hormone level (weighted mean difference, –1.56; 95% confidence interval, –2.44 to –0.69; I 2 = 22%). Heterogeneity between studies was low for studies using the Gen II and IOT anti-Müllerian hormone kits. One study used the new ultrasensitive anti-Müllerian hormone enzyme-linked immunosorbent assay assay and showed significant decline in circulating anti-Müllerian hormone.
Sensitivity analysis
Pooled analysis of 8 studies with a low risk of bias (as defined in previous text) including 297 patients showed a statistically significant fall in postoperative serum anti-Müllerian hormone concentration (weighted mean difference, –1.05; 95% confidence interval, –1.29 to –0.81; I 2 = 43%). Heterogeneity between studies was low.
Studies with ovarian cyst >5 cm
Seven studies including 276 patients were identified. Pooled analysis revealed a statistically significant fall in postoperative serum anti-Müllerian hormone concentration (weighted mean difference, –1.13; 95% confidence interval, –1.56 to –0.70; I 2 = 62%). Heterogeneity between studies was high.
Studies with different histological types
Analysis of 6 studies including 158 patients with dermoid cysts revealed a statistically significant fall in serum anti-Müllerian hormone concentration (weighted mean difference, –1.27; 95% confidence interval, –1.93 to –0.62; I 2 = 55%). Similarly, an analysis of 4 studies including 84 patients with cystadenomas showed a statistically significant decline in serum anti-Müllerian hormone concentration (weighted mean difference, –1.59; 95% confidence interval, –2.00 to –1.17; I 2 = 0%).
Secondary outcomes
Three studies measured changes in serum follicle-stimulating hormone concentrations, but only 2 (including 95 patients) of these provided full pre- and postoperative data. Pooled analysis of these 2 studies revealed no significant change in circulating follicle-stimulating hormone following ovarian cystectomy (weighted mean difference, –0.50; 95% confidence interval, –1.28 to 0.28; I 2 = 0%). The authors of the other study reported that follicle-stimulating hormone levels did not change after surgery, but they failed to present the actual data.
With regard to antral follicle count, although 1 study included this ovarian reserve marker as an outcome measure, the authors provided the postoperative antral follicle count data only and failed to present the preoperative values.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


