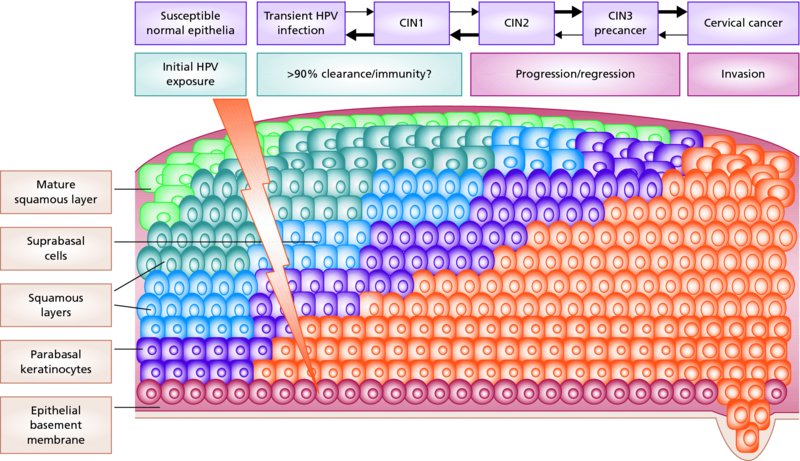
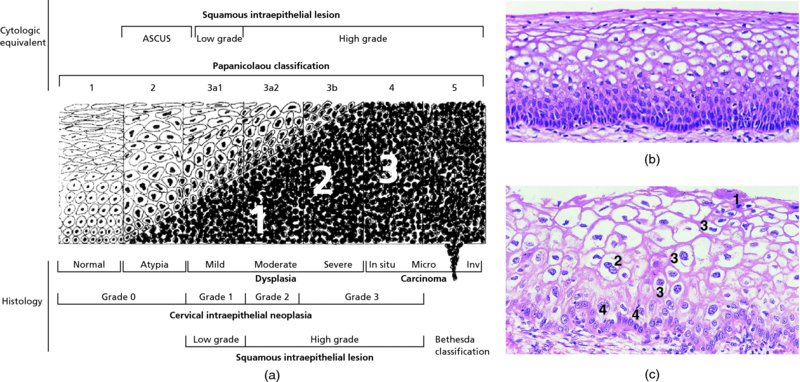
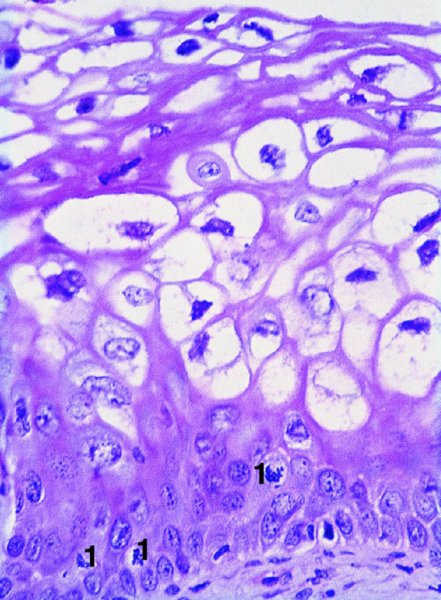
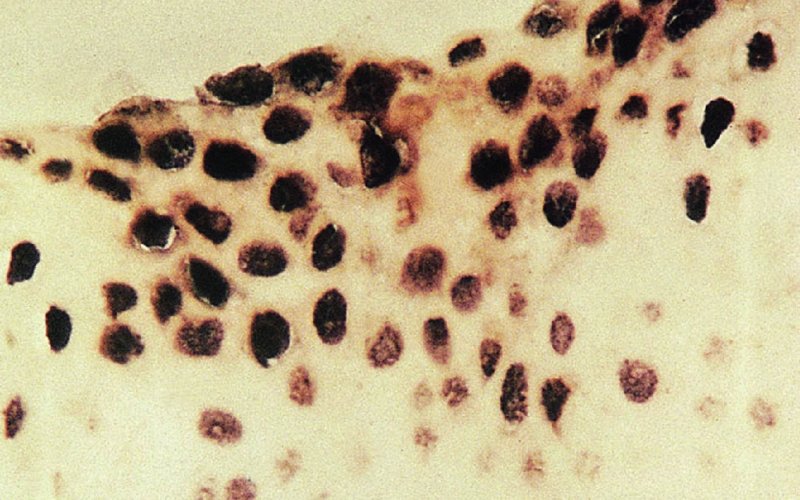
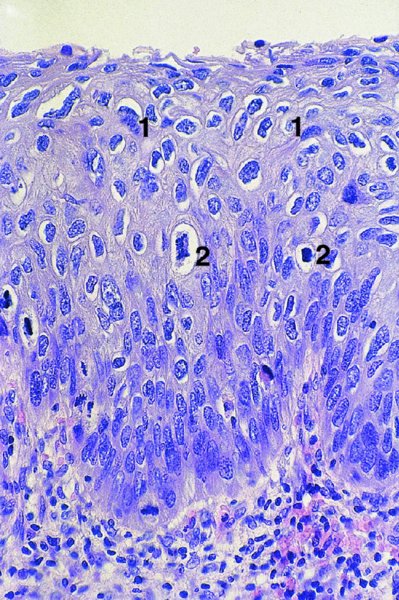
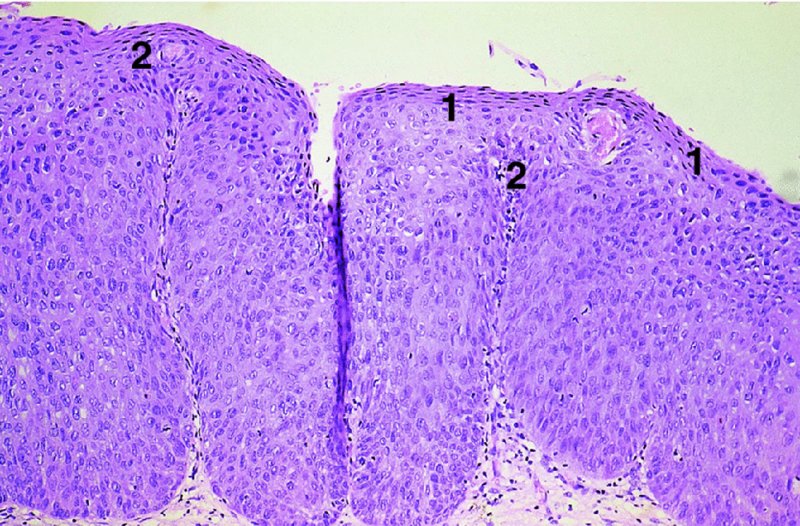
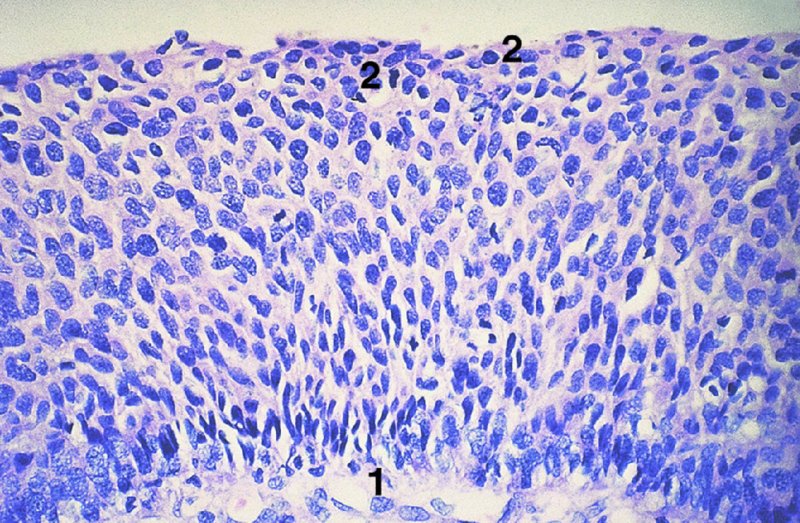
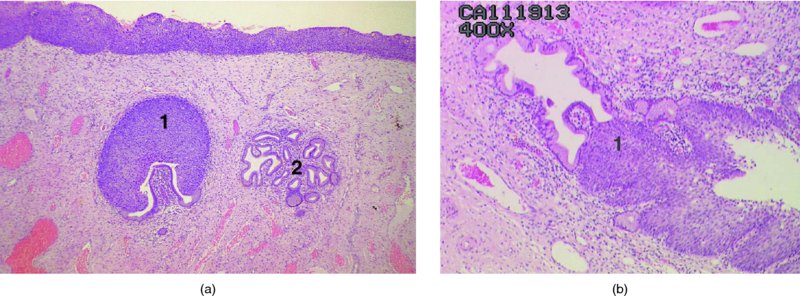
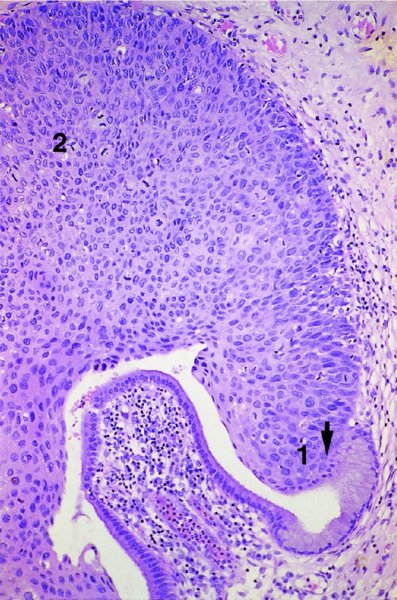
CHAPTER 1 Approximately 1 in 10 female cancers diagnosed worldwide are cancers of the cervix. Except in countries with effective screening programs the incidence has changed little. The discovery of cytologically detectable and anatomically confirmed premalignant phases has successfully shifted the presentation of cervical squamous tumors from the clinical to the preclinical stage; this being associated with dramatic falls in the prevalence of the disease when effective screening is undertaken. It is critical that the terminologies used for cytologic and histologic diagnosis are comparable so that they can be correlated with the colposcopic findings. In the last 15 years, our understanding of the molecular events associated with lower anogenital tract neoplasia has developed rapidly. In response to this knowledge, the terminology has changed with each new classification system providing a higher degree of sophistication. Gradually the terminology has become more uniform and a histologist’s implications and a clinician’s inferences have become more reliable. It is imperative that the nomenclature is uniform throughout the world for ease with statistics, although currently there still remain differences in terminologies between the USA and the rest of the world. The concept of cervical cancer precursors dates back to 1886 when Williams noticed, next to invasive cancers, areas of epithelium that he recognized as non-invasive. The term “carcinoma in situ” (CIS) was introduced by Broders in 1932, and this term has been used from its introduction to the present day. Smith and Pemberton in 1934 reported a relationship between CIS and invasive cancer when they found that the changes described as CIS by Broders were present in a retrospective review of biopsies from patients who subsequently developed invasive cancer. This combination of histologic observations and retrospective clinical analysis led to the concept that invasive squamous cell carcinoma develops from precursor lesions that can be identified by the pathologist (Figure 1.1). Figure 1.1 The concept of cervical cancer precursor progression and human papillomavirus infection. CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. With the advent of exfoliative cytology it was recognized that not all cervical abnormalities had full-thickness atypical changes as described for CIS. The lesions, which were morphologically less complex than CIS but still contained many cytologic and histologic features of that entity, were recognized to form a broad histologic spectrum, ranging from minimal deviation changes, which closely resembled the normal epithelium, through epithelia with increasingly severe atypia and disorganization to the classical CIS. Reagan and coworkers in 1956 introduced the term “dysplasia” to designate those abnormalities with histologic and cytologic features that were intermediate between normal epithelium and CIS. Following this, Walters and Reagan in 1956 subclassified the dysplasia into three groups—mild, moderate, and severe—depending upon the degree to which full thickness of the epithelium was replaced by the atypical cells. The higher the histologic grade, the more likely the lesion was to progress to invasive cancer and the higher the risk of developing cancer. When the terminology was originally proposed and generally accepted, the prevailing clinical management of patients with dysplasia and CIS was that patients with CIS required a hysterectomy to prevent the development of cancer. Women with a histologic diagnosis of dysplasia, whose clinical course was not well understood but could vary from remission to persistence and progression to CIS, were ignored, followed up, or treated by a variety of means, depending upon the clinician’s understanding and acceptance of the natural history data. However, the distinction between dysplasia and CIS was frequently based upon poorly defined and arbitrary histologic distinctions. It gradually became clear that the enormous differences in approaches to treatment were the result of the unreliability of the diagnoses because of the high interobserver variability. Figure 1.2 (a) Diagrammatic representation of the cytologic equivalents of cervical intraepithelial disease. (b) Normal cervical epithelium. The epithelium is uniform in pattern and cytology. The cells mature progressively as they move toward the surface, the nuclei become pyknotic, and glycogenation occurs. (c) Human papillomavirus infection “alone” (low-grade squamous intraepithelial lesion). There is mild, full-thickness, cellular enlargement and hyperchromasia with karyopyknosis (1), binucleation (2), and a raisin-like nucleus with a surrounding halo (3). In the bottom three cell layers there is variable nuclear and cell size, irregular variable condensed chromatin aggregation, degenerative nucleoplasmic clearing, nucleolar increase, and some visible early koilocytes (4). These features at (4) are typical of regenerative or hyperplastic epithelial layers with some degenerative or poorly preserved features added in (e.g., nuclear clearing). They are not the features of neoplasia. ASCUS, atypical glandular cells of undetermined significance. When patients with dysplasia were followed prospectively, it was noted that some cases regressed, some persisted, and some progressed to CIS. It also became clear that there was an inverse correlation of regression with histologic grade, and a direct correlation of progression with histologic grade. On the basis of these studies, a new term—“cervical intraepithelial neoplasia” (CIN) or squamous intraepithelial lesion (SIL)—was proposed. It was divided into grades 1, 2, and 3, in which CIN1/low-grade SIL (LSIL) corresponded to mild dysplasia, CIN2/high-grade SIL (HSIL) to moderate dysplasia, and CIN3/high-grade SIL(HSIL) corresponded to severe dysplasia and CIS. The concept of a continuum in the process of moving from normal epithelium through epithelial precursor lesions to invasive cancer was subsequently introduced, although this is now being challenged with many regarding CIN1/LSIL as no more than an expression of viral presence (human papillomavirus (HPV)) with minimal neoplastic potential. Over the last two decades through accumulated experimental, molecular, and clinical evidence it has become accepted that HPV is the etiologic agent in the majority of cervical and lower genital tract lesions. The realization has gradually emerged that, although the infections are ubiquitous in the young sexually active population, they are transient, often appearing and disappearing without cytologic abnormality. Persistent infection by high-risk HPV subtype is strongly associated with progression to high-grade lesions and invasion. Subsequent to the recognition of HPV and its association with cervical dysplasia a plethora of terms such as flat condyloma, condyloma planum, condylomatous atypia, koilocytotic atypia, and warty atypia came into use. Many claimed that the new observation of the cyto-/histologic evidence of HPV infection need not and should not influence the microscopic grading of CIN. All low- and high-risk HPV types are thought to be associated with identical low-grade histology. Conversely, it is not possible to determine the HPV subtype merely by histologic and cytologic observation. The overdiagnosis of CIN1/LSIL in the presence of flat condylomatous HPV changes has also been stressed. The convention has developed to report biopsies as showing HPV atypia without changes of CIN/SIL or HPV atypia and CIN/SIL combined. In reality, there is little disagreement that most mild dysplasias, HPV and CIN1 or HPV alone, and indeed the majority of moderate dysplasias, or HPV and CIN2, either spontaneously regress or stay the same after prolonged follow-up, regardless of the histopathologic segregation. The practical recognition of the limited reproducibility of cytologic and histologic assessments resulted in a new nomenclature: The Bethesda System (TBS) (National Cancer Institute Workshop, 1992). This terminology, which was initially aimed at cytology, was in parallel with the development in histopathologic terminology towards two grades of disease as described by Richart in 1990. TBS combines flat condylomatous (HPV) changes and low-grade CIN (CIN1) into LSIL; HSIL corresponds to CIN2 and CIN3. The term “lesion” is substituted for “neoplasia” since the morphologic grades of disease do not necessarily identify a neoplastic process. Conventionally, CIN2 and 3 are grouped together as both have the potential to progress to invasive disease. In the USA, TBS is applied to both histology and cytology. In most other countries CIN (1, 2, and 3) currently remains the most common terminology for histologic reporting; cytology, although using TBS in many centers, has the usage in others of the older terminology of mild, moderate, and severe dyskaryosis. This will be described in detail in Chapter 5. The principal alterations that characterize CIN include changes in the organization within the epithelium and in the cytomorphology of the cells. These changes appear to be triggered by HPV infection and represent the cytopathic effects of the virus in its replicative cycle. The morphologic changes occur because of the ability of the virus to interrupt the cell cycle, to induce polyploidy, and to inhibit functional cytoplasmic differentiation. This produces the characteristic “koilocytotic” change that is seen as enlargement of the bi- or multinucleated cells with a sharp perinuclear halo dispersed in a non-uniform “shotgun” pattern throughout the layers of squamous epithelium. These cells are hyperdiploid cells that have increased amounts of stainable nuclear material and hence are seen as hyperchromatic. Cells die and increased numbers of adjacent cells are recruited into the cycle to replace them. These cells exhibit nuclear enlargement, exaggerating previously unnoticed nuclear envelope irregularities. The most important changes at the tissue level are a retardation of the progressive maturation through the vertical layers of the normal epithelium (Figure 1.2b) and the expansion of the proliferating parabasal or suprabasal zone associated with the presence of koilocytes (the pathognomonic cytopathic change of HPV-infected cells). In low-grade lesions the atypically proliferating suprabasal cells occupy the lower third of the epithelial thickness, though the cytopathic changes of HPV infection are full thickness. The presence or absence of CIN relies on the presence of morphologically recognizable neoplastic cellular features in the suprabasal population (Figure 1.2c). Polyploidy in such lesions is further evidenced by bi-, tri-, or multinucleation, which is another important feature of virally infected epithelium. Figure 1.3 Human papillomavirus infection and cervical intraepithelial neoplasia 1 (low-grade squamous intraepithelial lesion). The same features are present in the top three-quarters of the epithelium as in Figure 1.2b. Now, however, the bottom four layers show more vital crowded cells with open chromatin. The additional feature is visible mitoses (1). Figure 1.4 Cervical intraepithelial neoplasia with in situ hybridization for high-risk human papillomavirus (HPV) DNA. Note that the nuclei are overlaid with reaction product, beginning about midway in the epithelium. The nuclei that are “positive” contain a minimum of about 50 copies of HPV per cell (the minimum sensitivity level for this test). The observer should look for the characteristic features of HPV infection in a biopsy and then for the presence or absence of “disturbed” suprabasal layers that might allow the separation of such lesions into HPV alone or HPV and CIN1 (Figures 1.3, 1.4). Within TBS all such lesions are ascribed to LSIL without further differentiation. A pragmatic approach to treatment exists variably throughout the world in response to whether the precursor lesion is termed “low grade” or “high grade” after biopsy. In countries where CIN terminology is operative there has been considerable variation and difference in the placement of CIN2 into one or other of these categories. If CIN2 is considered high grade, then treatment is largely obligatory. In some countries (e.g., the USA) the grouping of CIN2 lesions with CIN3 in HSIL probably has fewer practical implications since both HPV/CIN1 (LSIL) and CIN2 or 3 (HSIL) are predominantly treated on consideration of the colposcopic extent or findings. Figure 1.5 Intermediate-grade cervical intraepithelial neoplasia (CIN2; high-grade CIN; high-grade squamous intraepithelial lesion) and human papillomavirus infection. The changes are much more florid than those seen in Figure 1.3 with proliferation of basal-type cells. Koilocytotic cells are confined to the upper half (1), but “truly” neoplastic cells, with nuclei demonstrating coarse-pepper nuclear chromatin increases without conspicuous nucleoli, are present, together with mitoses, up to half way from the base (2). Figure 1.6 High-grade cervical intraepithelial neoplasia (CIN3; high-grade squamous intraepithelial lesion). Full-thickness atypia, with proliferation of suprabasal cells to probably three-quarters of the height within the epithelium followed by superficial maturation and flattening and minimal koilocytosis (1). There is a papillary configuration of the epithelium with high vessels (2). Figure 1.7 Cervical intraepithelial neoplasia 3 (CIN3; high-grade squamous intraepithelial lesion (HSIL)). These appearances are full-thickness effacement by “small cell,” suprabasal cells exhibiting characteristic neoplastic coarse-pepper chromatin without conspicuous macronucleation. This is a basaloid variant of CIN3 (HSIL). There is hardly detectable surface differentiation and thus no koilocytotic change. Mitoses are present close to the surface (2). Figure 1.8 (a) Crypt involvement by cervical intraepithelial neoplasia 3 (CIN3; high-grade squamous intraepithelial lesion (HSIL)). At (2) benign uninvolved crypt profiles are seen. (b) A CIN3 (HSIL) lesion (1) extending into a cervical crypt. Areas of glandular epithelium are seen towards the apex of the crypt. Figure 1.9 Higher power view of involved crypt. Note the pushing edge of the cervical intraepithelial neoplasia 3 (high-grade squamous intraepithelial lesion) margin at (1) lifting (arrowed) the attenuated and degenerating endocervical cell layer off its basement membrane. There are numerous mitoses including tripolar metaphases (2). The histologic assessment of whether the epithelial changes involve up to two-thirds of the epithelium or are practically full thickness will allow the designation of CIN2 or 3 accordingly. The recognition of cytopathic HPV-related changes in the upper squamous layers decreases correspondingly. In high-grade CIN (HISL/CIN2 or 3) there is more hyperchromasia; the mitoses occur throughout the full epithelial thickness; the polarity of the parabasal cells is substantially altered; and a high degree of disorganized maturation and cytologic atypia is evident. These changes are thought to correspond with the occurrence of viral integration and/or amplification in cell nuclei and the development of aneuploidy, namely abnormal chromosome numbers and DNA quantities. Along with more severe nuclear chromatin changes come abnormal and bizarre multipolar mitoses. Whether or not these changes are clonal (i.e., arising in a single daughter cell line) has not been determined. Clonality may be essential, however, to terming any lesion “neoplastic,” since its occurrence demonstrates a survival advantage of the proliferating clone and the effacement of less genetically favored populations. Among apparently similar histopathologic high-grade lesions only a certain, but unknown, proportion may be predominantly monoclonal or neoplastic. Disease may also extend into the cervical crypts that are potentially found under any premalignant lesion within the transformation zone (Figures 1.8a,b, 1.9). Recognition as to the depth of crypt involvement of such lesions is essential for their complete removal.
The histopathology of lower genital tract neoplasia
1.1 Introduction
1.2 Terminology
The concept of cervical cancer precursors
Carcinoma in situ
Terminology and management (Figure 1.2a)
Biology and natural history
The role of human papillomavirus
Nomenclature of human papillomavirus lesions
Rationalization of histologic terminology
Low- and high-grade squamous intraepithelial lesions
Present nomenclature
1.3 Histopathologic features of cervical intraepithelial neoplasia or squamous intraepithelial lesion
Low-grade disease (mild dysplasia, cervical intraepithelial neoplasia 1, human papillomavirus/cervical intraepithelial neoplasia 1, low-grade squamous intraepithelial lesion) (Figures 1.2, 1.3, 1.4)
High-grade disease (moderate or severe dysplasia, carcinoma in situ, cervical intraepithelial neoplasia 2 or 3, high-grade squamous intraepithelial lesion) (Figures 1.5, 1.6, 1.7, 1.8, 1.9)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


