Background
Myoinositol and D-chiroinositol improve insulin resistance in women with obesity and gestational diabetes and in postmenopausal women with metabolic syndrome. We previously reported that offspring born to hypertensive dams lacking endothelial nitric oxide synthase and fed a high-fat diet develop metabolic-like syndrome phenotype.
Objective
The objective of the study was to investigate the effect of a mixture of myoinositol/D-chiroinositol supplementation during pregnancy on the maternal metabolic profile in pregnancies complicated by the metabolic-like syndrome and obesity using a pregnant mouse model.
Study Design
Female heterozygous endothelial nitric oxide synthase –/+ mice with moderate hypertension were placed on a high-fat diet for 4 weeks to induce a metabolic-like syndrome phenotype. Similarly, wild-type C57BL/6 mice were placed on a high-fat diet for 4 weeks to induce a murine obesity model. Mice were then bred with wild-type males. On gestational day 1, dams were randomly allocated to receive either a mixture of myoinositol/D-chiroinositol in water (7.2/0.18 mg/mL, respectively) or water as control (placebo). At term (gestational day 18), maternal weights, systolic blood pressure, and a glucose tolerance test were obtained. Dams were then killed; pups and placentas were weighed and maternal blood collected. Serum levels of metabolic biomarkers relevant to diabetes and obesity (ghrelin, gastric inhibitory peptide, glucagon-like peptide 1, glucagon, insulin, leptin, resistin) were measured by a multiplex enzyme-linked immunosorbent assay. Analysis was done comparing metabolic-like syndrome-myoinositol/D-chiroinositol–treated vs metabolic-like syndrome–nontreated mice and obese-myoinositol/D-chiroinositol–treated vs obese nontreated mice.
Results
Mean systolic blood pressure was lower in metabolic-like syndrome pregnant mice treated with myoinositol/D-chiroinositol compared with placebo ( P = .04), whereas there was no difference in systolic blood pressure between treated and placebo-treated obese pregnant mice. Pregnant metabolic-like syndrome mice treated with myoinositol/D-chiroinositol showed lower glucose values during the glucose tolerance test and in the area under the curve (myoinositol/D-chiroinositol: 17512.5 ± 3984.4 vs placebo: 29687.14 ± 8258.7; P = .003), but no differences were seen in the obese pregnant mice. Leptin serum levels were lower in the metabolic-like syndrome-myoinositol/D-chiroinositol–treated mice compared with the placebo group (myoinositol/D-chiroinositol: 16985 ± 976.4 pg/dL vs placebo: 24181.9 ± 3128.2 pg/dL, P = .045). No other differences were seen in any of the remaining serum metabolic biomarkers studied in metabolic-like syndrome and in obese pregnant mice. Maternal weight gain was not different in the pregnant metabolic-like syndrome dams, whereas it was lower in the obese myoinositol/D-chiroinositol–treated dams compared with the placebo group (myoinositol/D-chiroinositol: 10.9 ± 0.5 g vs 12.6 ± 0.6 g, P = .04). Fetal and placental weights did not differ between myoinositol/D-chiroinositol–treated and nontreated pregnant dams with metabolic-like syndrome and obesity.
Conclusion
Combined inositol treatment during pregnancy improves blood pressure, glucose levels at the glucose tolerance test, and leptin levels in pregnant dams with metabolic-like syndrome phenotype but not in obese pregnant dams. In addition, inositol treatment was associated with lower gestational weight gain in the obese but not in the metabolic-like syndrome pregnant dams.
Metabolic syndrome and obesity are growing causes of morbidity and mortality worldwide. Metabolic syndrome is a major risk factor for cardiovascular disease and is defined by the National Institutes of Health by having at least 3 of the following conditions: hypertension, elevated fasting plasma glucose, central obesity, elevated triglycerides, or low high-density lipoprotein cholesterol.
Metabolic syndrome etiopathogenesis and long-term consequences are still largely unknown, and preventive strategies have not yet been identified. The impact of metabolic syndrome and obesity during pregnancy is substantial: indeed, all metabolic changes that develop during pregnancy have well-known effects on not only maternal and fetal health during pregnancy, but they also act as a catalyst for future health throughout later life.
Epidemiological and animal studies have shown that pregnancies complicated by metabolic syndrome and obesity are at risk for premature cardiovascular disease, gestational diabetes, and preeclampsia and predispose the offspring to an increased risk of cardiovascular and metabolic disease later in life.
Inositols are a family of simple carbohydrates naturally found in several foods. Inositols exist in 9 possible stereoisomers, 2 of which are predominantly found in eukaryotic cells: myoinositol and D-chiroinositol. The exact molecular mechanisms of action of myoinositol and D-chiroinositol have not yet been fully elucidated; however, it is well established that myoinositol and D-chiroinositol have different roles as mediators of insulin, which lead to different functions within the cells.
Myoinositol is the precursor of inositol triphosphate, a second messenger, responsible for the regulation of many hormones such as insulin, thyroid-stimulating hormone, and follicle-stimulating hormone. The activation of phospholipid-containing myoinositol by insulin activates glucose transporters, such as glucose transporter-4, and increases the cell membrane permeability to glucose, which gets into the cell and is immediately available as substrate.
D-chiroinositol is the conversion product of myoinositol, by an epimerization reaction, which is unidirectional and insulin dependent. D-chiroinositol, different from myoinositol, is able to determine the intracellular accumulation of glucose (ie, glycogen synthesis).
So both stereoisomers are considered to play a key role in the insulin pathway, acting synergistically as insulin-sensitizing agents through the enhancement of glucose peripheral tissue uptake and glycogen synthesis. In vivo animal and human studies demonstrated that inositol supplementation during pregnancy improve the glucose profile and reduces the adverse effects of hyperglycemia. Moreover, myoinositol supplementation was proven to reduce insulin resistance in postmenopausal women with metabolic syndrome and in women with polycystic ovary syndrome, a metabolic and endocrine disorder associated with insulin resistance.
However, to the best of our knowledge, the inositol supplementation effect has never been investigated in pregnancies complicated by metabolic syndrome. Moreover, most of the studies in pregnant women focus on the effect of myoinositol supplementation alone, whereas mounting evidence in polycystic ovary syndrome studies suggest that the administration of combined myoinositol/D-chiroinositol at the physiological plasma ratio (40:1) ensures better clinical results, such as the reduction of insulin resistance and cardiovascular risk parameters.
Thus, our aim was to evaluate the effect of natural compounds, such as myoinositol and D-chiro inositol, used as a mixture, to treat pregnancies complicated by obesity and metabolic syndrome, which represent different degrees of a similar metabolic disorder. We hypothesized that myoinositol/D-chiroinositol treatment improves the abnormal maternal metabolic profile in a pregnant mouse model of either metabolic-like syndrome or obesity. The metabolic profile improvement seen with myoinositol/D-chiroinositol treatment could also translate into positive long-term maternal and fetal health.
To achieve our aim, we used well-characterized mice models of obesity and metabolic syndrome that will allow identifying the best target population for a myoinositol/D-chiroinositol treatment in pregnancies with metabolic abnormalities. We previously reported that heterozygous mice lacking endothelial nitric oxide synthase gene and born to hypertensive mothers, endothelial nitric oxide synthase –/+ , when fed a high-fat diet for at least 4 weeks, develop metabolic-like syndrome phenotype such as obesity, glucose intolerance, elevated systolic blood pressure, low high-density lipoprotein, high insulin, and low adiponectin levels compared with their counterpart fed a control diet. Furthermore, wild-type C57BL/6J female mice fed an obesogenic diet from weaning for 4 consecutive weeks show diet-induced obesity.
Materials and Methods
Animals
Female and male mice, homozygous for disruption of the eNOS gene (endothelial nitric oxide synthase-knockout –/– , strain B6.129P2, stock number 002684) and their age-matched wild-type controls (strain C57BL/6J, stock number 000664) were purchased from Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age. The study was approved by the Animal Welfare Committee (AWC) of the University of Texas Health Science Center at Houston. The mice were housed separately in temperature- and humidity-controlled quarters with constant 12:12-hour light-dark cycles in the animal care facility at the University of Texas Health Science Center at Houston.
Metabolic-like syndrome mouse model
Endothelial nitric oxide synthase-knockout –/– females were bred with wild-type males to obtain endothelial nitric oxide synthase +/– heterozygous females. Then a metabolic-like syndrome mouse model was achieved by using endothelial nitric oxide synthase –/+ heterozygous offspring manifesting a moderate hypertension phenotype and fed an obesogenic diet for 4 consecutive weeks after weaning ( Figure 1 ).
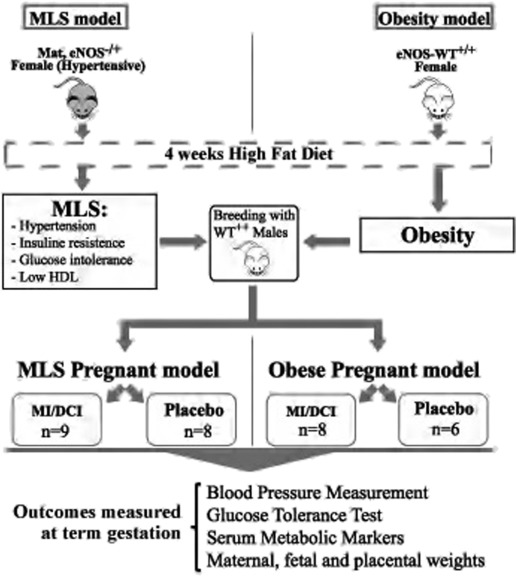
At 7–8 weeks of age, nonpregnant endothelial nitric oxide synthase –/+ females were bred with wild-type males. At gestational day 1 of pregnancy, determined by the presence of a vaginal plug after overnight exposure to male breeders, metabolic-like syndrome dams were randomly allocated to receive either a mixture of myoinositol/D-chiroinositol dissolved in water (see next paragraph for concentrations) or plain water, as placebo (control group) ( Figure 1 ).
Obesity mouse model
To induce the obesity phenotype ( Figure 1 ), wild-type–C57BL/6J female mice were fed an obesogenic diet from weaning for 4 consecutive weeks. Then at 7–8 weeks of age, obese females were bred with wild-type males and starting from gestational day 1, obese mice were randomly allocated to receive either a mixture of myoinositol/D-chiroinositol dissolved in water (see next paragraph for concentrations) or plain water, as placebo (control group).
Obesogenic diet
Both models, endothelial nitric oxide synthase –/+ –heterozygous and wild-type females offspring, were allocated to an obesogenic diet, high-fat diet (D12492, 60% of fat) until 7–8 weeks of life to induce the metabolic-like syndrome and obesity phenotypes, respectively. The high-fat diet was maintained during the whole pregnancy until animals were killed at term, on gestational day 18. Daily food consumption was estimated by subtracting the total amount of food left on the grid from the initial weight of food supplied.
Inositol supplementation during pregnancy
The myoinositol/D-chiroinositol mixture corresponds to the physiological plasma ratio of myoinositol and D-chiroinositol equal to 40:1, which has been proven to be the most effective. On gestational day 1, metabolic-like syndrome and obese mice were randomly allocated to receive either a mixture of myoinositol/D-chiroinositol dissolved in water (7.2/0.18 mg/mL, respectively, based on previous animals and human studies) or plain water as placebo.
Considering that blood volume during pregnancy physiologically shows a 20% increase compared with the nonpregnant status, we adjusted the myoinositol/D-chiroinositol doses by a 20% increase to the previously established doses used in a nonpregnant obese mouse model (myoinositol: 6 mg/mL). Myoinositol and D-chiroinositol were purchased from Sigma Chemicals (St Louis, MO).
The treatment was maintained until term gestation (gestational day 18 of pregnancy), when dams were killed. Pregnant mice were single housed to be able to carefully evaluate water intake and consequently the daily dose of myoinositol/D-chiroinositol. Daily water consumption was estimated by subtracting the total amount of water left in the bottle from the initial amount supplied. On average, pregnant mice drink 5 mL/day, so myoinositol/D-chiroinositol daily consumption was approximately 36/0.9 mg of, respectively, myoinositol/D-chiroinositol per day per mouse.
At term gestation, the metabolic profile of the metabolic-like syndrome and obese pregnant dams were obtained including the following: systolic blood pressure, a glucose tolerance test, maternal gestational weight gain, and serum levels of metabolic biomarkers relevant to diabetes and obesity. Damns were then killed and pups and placentas weights and numbers collected.
Blood pressure measurement
Systolic blood pressure measurements were always taken on gestational day 18 in the morning at the same time using a calibrated, 8-chamber, tail-cuff system (CODA; Kent Scientific, Torrington, CT). Dams were kept warm using a warming pad. Mice underwent an initial 10 cycles of acclimatization period; blood pressure was then monitored and recorded over 10 new cycles. Systolic blood pressure averaged of the last 10 cycles was used for the final blood pressure measurement and utilized for data analysis.
Glucose tolerance test
Pregnant dams on gestational day 18 underwent a glucose tolerance test after being fasting for 6 hours. Mice received 1.0 g/kg of glucose intraperitoneally, and serial blood glucose levels, via a tail nick at 0, 15, 30, 60, and 120 minutes, were immediately determined with the Accu-Chek Aviva blood glucose meter system (Roche Diagnostics, Indianapolis, IN) after glucose administration. Glucose levels and the area under the curve were compared between the metabolic-like syndrome and obese pregnant mice treated with myoinositol/D-chiroinositol and identical nontreated mice.
Serum metabolic panel
Blood samples were obtained as soon as animals were killed by heart puncture. Serum levels of metabolic biomarkers relevant to diabetes and obesity such as glucagon, insulin, leptin, ghrelin, gastric inhibitory peptide, glucagon-like peptide 1, and resistin were measured by a multiplex enzyme-linked immunosorbent assay (Bio-Rad Laboratories, Hercules, CA).
Statistical analysis
Results are expressed as mean ± SEM. All the data were analyzed using an unpaired t test with SigmaPlot 12. The analysis was done comparing metabolic-like syndrome myoinositol/D-chiroinositol–treated pregnant mice vs metabolic-like syndrome nontreated (placebo) pregnant mice and obese myoinositol/D-chiroinositol–treated pregnant vs obese nontreated (placebo) pregnant mice. Numbers of pregnant mice per group were as follows: metabolic-like syndrome myoinositol/D-chiroinositol treated, n = 9; metabolic-like syndrome placebo treated, n = 8; obese myoinositol/D-chiroinositol treated, n = 8; and obese placebo treated, n = 6. ( Figure 1 ).
Results
Food and water intake
Daily food intake was not different between metabolic-like syndrome myoinositol/D-chiroinositol–treated and metabolic-like syndrome–placebo mice (myoinositol/D-chiroinositol: 6.0 ± 0.9 g vs placebo: 6.5 ± 0.7 g) or between obese myoinositol/D-chiroinositol–treated and obese placebo-treated (myoinositol/D-chiroinositol: 6.3 ± 0.6 g vs placebo: 6.0 ± 0.7 g). Daily water intake was not different between metabolic-like syndrome-myoinositol/D-chiroinositol–treated and metabolic-like syndrome–placebo mice (myoinositol/D-chiroinositol: 5.1 ± 0.9 mL vs placebo: 4.9 ± 0.7 mL) or between obese myoinositol/D-chiroinositol–treated and obese placebo-treated (myoinositol/D-chiroinositol: 4.8 ± 0.9 g vs placebo: 5.0 ± 0.7 g).
Systolic blood pressure measurement
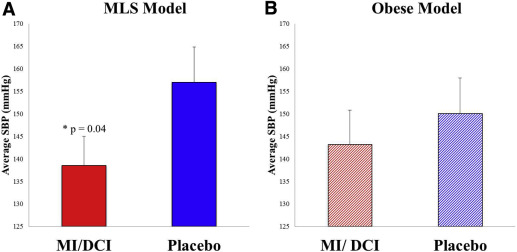
At term gestation, mean systolic blood pressure was lower in metabolic-like syndrome pregnant mice treated with myoinositol/D-chiroinositol compared with the placebo group (myoinositol/D-chiroinositol: 138.52 ± 6.48 mm Hg vs placebo: 157.03 ± 7.79 mm Hg, P = .04) ( Figure 2 A). In the obese pregnant dams, there was no difference in systolic blood pressure between myoinositol/D-chiroinositol–treated and placebo damns (myoinositol/D-chiroinositol: 143.18 ± 7.6 vs placebo: 150.09 ± 7.9) ( Figure 2 B).
Glucose tolerance
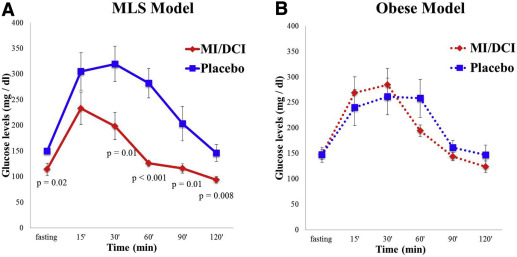
The glucose levels in the glucose tolerance test were lower in the metabolic-like syndrome pregnant mice treated with myoinositol/D-chiroinositol compared with placebo-treated mice at all time periods except for the 15 minute value ( Figure 3 A). To confirm, the area under the curve for the glucose tolerance test was lower in metabolic-like syndrome mice myoinositol/D-chiroinositol–treated group (myoinositol/D-chiroinositol: 17512.5 ± 3984.4 vs placebo: 29687.14 ± 8258.7; P = .003). In contrast, in the obese pregnant dams, the glucose levels in the glucose tolerance test and the area under the curve were not different between myoinositol/D-chiroinositol–treated and placebo-treated dams (myoinositol/D-chiroinositol: 23573.6 ± 4758.2 vs placebo: 25410 ± 5764.4) ( Figure 3 B).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


