The Ear
John H. Greinwald Jr., Margaret A. Kenna, and Blake C. Papsin
THE NORMAL EAR
 ANATOMY
ANATOMY
The ear is divided into the external, middle, and inner ear compartments (Fig. 369-1). The external ear consists of the auricle and the external auditory canal. The primary function of the auricle is to channel sound energy toward the middle ear conducting apparatus. The lateral opening of the external auditory canal is the external meatus, which is bordered medially by the tympanic membrane. The lateral one third of the external canal is cartilaginous, and the medial two thirds are bony. The canal is lined by skin that possesses cerumen glands and other adnexal structures (hair follicles, sebaceous glands) in its lateral half.
The normal tympanic membrane (Fig. 369-2) seals the opening between the external auditory canal and the middle and inner ear. The portion of the tympanic membrane inferior to the short process of the malleus (pars tensa) is a three-layered structure composed of a medial mucosal epithelium continuous with the middle ear mucosa, a middle fibrous tissue layer, and, finally, a lateral surface of squamous epithelium continuous with the external ear canal skin. The region of the tympanic membrane superior to the short process of the malleus (pars flaccida) does not have a middle fibrous layer, which is clinically significant because it allows the development of retraction pockets and acquired cholesteatomas.
The middle ear compartment is an aerated cavity that houses the three ossicles: malleus, incus, and stapes. The function of the ossicles is to efficiently transmit sound energy to the inner ear. The middle ear is connected to the nasopharynx anterosuperiorly via the eustachian tube. Posterior and superiorly, the middle ear cavity is connected to the mastoid air cell system by means of the mastoid antrum. These connections provide a pathway for extension of middle ear infection into the mastoid. This can cause coalescent mastoiditis.
The inner ear is divided into an auditory portion (the cochlea), a vestibular portion (three semicircular canals, the utricle, and the saccule), and the endolymphatic apparatus (the endolymphatic duct and sac). The cochlea is a coiled structure that houses the machinery responsible for transducing sound energy into neural impulses. The actual transducers are hair cells, which are precisely arranged in the organ of Corti. The organ of Corti, in turn, rests on the basilar membrane, which resonates in response to the incoming acoustic stimuli. The cochlea maintains a very specific fluid balance. The endolymph has a composition similar to intracellular fluid (ie, high potassium and low sodium), whereas the perilymph has a composition similar to that of extracellular fluid (ie, low potassium and high sodium). The establishment of this electrochemical gradient is critical for signal transduction and hearing. Clinical disorders that perturb this fluid homeostasis result in hearing loss and/or balance dysfunction.
The semicircular canals are oriented at approximately 90 degrees to each other, allowing detection of angular head motion in any given plane. Hair cells within the sensory portions of the semicircular canals are deflected in response to head movement in a particular plane of motion. The utricle and saccule represent organs for the detection of linear or gravitational acceleration. Congenital deformities affecting only the vestibular portion of the inner ear are rare but have been reported in association with other ear anomalies such as Goldenhar syndrome. Children with congenital vestibulopathy usually present with a delay in development of motor skills.
The endolymphatic duct and sac are believed to play a role in the maintenance of the specific fluid homeostasis of the inner ear. The vestibular aqueduct serves as the bony channel that transmits the endolymphatic duct and sac. Abnormalities of the endolymphatic sac are found in patients with Pendred syndrome. Enlarged vestibular aqueduct (EVA) is the most common bony abnormality of the inner ear and is present in about 25% of patients with sensorineural hearing loss (SNHL). The presence of an EVA has also been linked to hearing loss following relatively mild head trauma in children.
 EMBRYOLOGY
EMBRYOLOGY
At approximately 6 weeks of gestation, the mesoderm of the first and second branchial arches condense, giving rise to six hillocks of His. These hillocks are responsible for the formation of the auricle, which is mature in shape by 20 weeks. The external auditory canal is initially a solid core of ectoderm that invaginates medially at about 8 weeks. This core then undergoes resorption and canalization to leave a tubelike structure by 28 weeks. The most medial ectoderm remains intact to serve as the surface epithelium of the tympanic membrane. Congenital microtia reflects an abnormal development process and is estimated to occur in up to 1 in 20,000 births. Atresia of the external auditory canal similarly points toward a development problem, typically believed to occur during the end of the first or beginning of the second trimester.
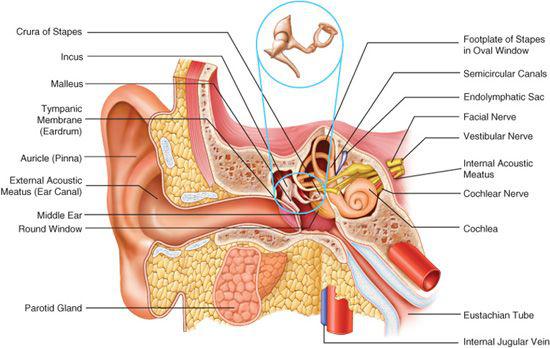
Figure 369-1. Anatomy of the ear: external, middle, and inner components.
The eustachian tube forms from the space between the second arch and the first pharyngeal pouch (pharynx). The middle ear cavity then develops from an outpouching at the lateral end of the eustachian tube primordium. The mastoid air cell system begins with the antrum. The middle ear system is filled with mucoid mesenchyme and secretions until close to the time of birth. The malleus and incus are derived from first and second branchial arch mesoderm and begin ossification as early as 16 weeks of gestation, when they are already of adult size. Auricular malformations such as microtia are of clinical relevance. Because the auricle forms early, it may be associated with concomitant malformation of the middle ear and mastoid. In contrast, a normal auricle with canal atresia suggests a developmental defect occurring around 28 weeks, a time when the ossicles and middle ear are already formed.
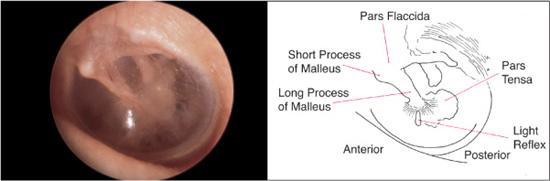
Figure 369-2. Normal tympanic membrane anatomy and landmarks. (Source: Knoop KJ, Stack LB, Storrow AB, Thurman RJ. The Atlas of Emergency Medicine. 3rd ed. New York: McGraw-Hill; 2010. Photo contributor: Richard A. Chole, MD, PhD.)
The inner ear develops from a thickening of the surface ectoderm, the otic placode, and forms the primitive otocyst at approximately 4 weeks of gestation. This otocyst forms the membranous labyrinth, including the cochlea, vestibular structures, and endolymphatic apparatus. By 6 weeks, the semicircular canals are well formed, and by 12 weeks, the cochlea completes its two and a half turns. Ossification of the inner ear structures begins at 15 weeks and is typically complete by 23 weeks of gestation. With the exception of the endolymphatic apparatus, the inner ear is approximately adult size by the end of 24 weeks.
 PHYSIOLOGY
PHYSIOLOGY
The length and shape of the external auditory canal imparts a particular optimal resonance frequency that affects an individual’s maximum sensitivity to sound. For most people, the ear demonstrates maximum sensitivity in the range of speech frequencies. The tympanic membrane and ossicles then further refine that sound energy conduction by means of focusing the energy onto the oval window and by employing a lever mechanism that confers a mechanical advantage to the system. Once the sound energy reaches the cochlea, it causes a specific portion of the basilar membrane to vibrate according to the frequency of the sound. This resonance of the basilar membrane then results in deflection of a specific set of hair cells and generation of an action potential. The “tonotopic” or frequency-specific arrangement of the basilar membrane and the cochlear hair cells is mirrored in the central auditory pathway of the brainstem and brain, where specific neurons are activated depending on the frequency of sound detected.
In the vestibular system, deflection of hair cells again forms the basis for signal transduction. Flow of endolymph either toward or away from the vestibule causes either an increase or decrease in firing rate of vestibular sensory cells, depending on which particular semicircular canal is involved.
EVALUATION OF THE AUDITORY AND VESTIBULAR SYSTEMS
 HISTORY
HISTORY
Behaviors such as inattentiveness, constant use of an inappropriately loud voice, excessive volume on televisions or radios, or even difficulty in classrooms can all be indicators of hearing loss. The onset and duration of hearing losses should be noted because the workup and management of congenital and acquired hearing losses differ. Associated symptoms such as pain, pressure, or drainage from the ears may also be significant features guiding the differential diagnosis. Symptoms of dizziness in the child are difficult to elicit and evaluate but if suspected they should be evaluated in order to rule out an inner ear vestibular disorder. Occasionally, the child with vestibular pathology presents with a developmental delay in motor skills or ambulation. A thorough history should also include inquiries into possible trauma. Foreign bodies in the ear canal or insertion of small objects into the ear can damage the tympanic membrane or even the ossicular chain, possibly resulting in hearing loss.
Social factors such as daycare attendance and exposure to second-hand smoke have been linked to an increased incidence of otitis media with effusion with hearing loss. A family history of hearing loss, particularly if in first-degree relatives is important when considering genetic causes of hearing loss.
Although universal newborn hearing screening is the standard across the United States, a complete history for the auditory system should explore prenatal and perinatal complications such as preeclampsia, infections including cytomegalovirus and toxoplasmosis, premature delivery, low Apgar scores, hyperbilirubinemia, low birth weight, neonatal intensive care unit admission, and ventilatory support.1 All are associated with an increased risk of hearing loss. At present, most newborn hearing screening programs use the auditory brainstem response (ABR) and/or otoacoustic emissions (OAEs) to evaluate those children at risk. (These are described later in this chapter.)
 PHYSICAL EXAMINATION
PHYSICAL EXAMINATION
Evaluation of the auditory system begins with observation of the overall craniofacial appearance. Shape of the auricle, position of the auricle, or any associated skin tags or preauricular pits suggest a possible abnormality of the auditory system. One of the more common causes of hearing loss in children is an accumulation of cerumen. A thorough exam of the ear requires gentle removal of any debris in the external auditory canal. Pulling the auricle posteriorly and superiorly can straighten the ear canal to facilitate examination.
A pneumatic otoscope is used to examine all structures from the external meatus to the tympanic membrane. Careful choice of the largest speculum that can comfortably fit within the child’s ear also facilitates this examination. The normal anterior canal wall of the external auditory canal demonstrates a “bulge” that obscures the anterior one third to one half of the tympanic membrane. To fully view the tympanic membrane, it is necessary to position the tip of the speculum accurately at this juncture and to angle the examiner’s view in an anterior direction.
The skin of the external meatus and external auditory canal should be surveyed for erythema, edema, lesions, or drainage. A reddened and swollen external canal with clear or purulent drainage suggests an external otitis, commonly referred to as “swimmer’s ear,” or secondary infection from middle ear drainage. The tympanic membrane should be evaluated for its overall integrity, color, vascularity, translucency, and mobility. Particularly significant areas to examine include (1) the superior part of the tympanic membrane (pars flaccida), where retraction pockets and acquired cholesteatomas frequently originate; (2) the anterior tympanic membrane, an area medial to which congenital cholesteatomas are noted; and (3) the remaining pars tensa, which is the most readily visualized portion of the tympanic membrane.
The tympanic membrane is normally a pale white or grayish structure. An erythematous tympanic membrane most commonly suggests an inflammatory process involving either the middle ear space or the drum itself. Subtle color changes in the tympanic membrane may suggest underlying vascular abnormalities of the middle ear. A bluish hue to the tympanic membrane can indicate a high jugular bulb protruding into the middle ear space, and a reddish coloration or mass seen in the tympanic membrane might raise the suspicion of an anomalous internal carotid artery in the middle ear. The vascularity of the tympanic membrane itself can also be an indicator of the status of the tympanic membrane and the middle ear space. The normal partial translucency provides some indication of the status of the middle ear structures and space. In the healthy ear, the short process, manubrium, and umbo of the malleus are readily visible (Fig. 369-2). The shadow or outline of the incus and the dense bone of the promontory are also discernible. Middle ear effusions, thickening of the tympanic membrane, or other middle ear pathology such as cholesteatoma may obscure these structures. The integrity of the tympanic membrane should also be assessed. Finally, with a speculum that is large enough to seal off the external auditory canal, a pneumatic otoscope should be used to gently move the tympanic membrane with a puff of air. Decreased mobility of the tympanic membrane is most commonly a result of fluid in the middle ear space and can be one of the most useful clinical findings for diagnosing otitis media with effusion.2
 TESTS OF FUNCTION
TESTS OF FUNCTION
Audiometry provides a quantitative measure of hearing and functionally assesses the entire auditory system. A trained audiologist uses one or more of a battery of testing techniques available, depending on the child’s age and development. All children with suspected or confirmed ear pathology, suspected congenital hearing loss, or a delay in the development of communication skills should undergo formal audiologic testing as early as possible. Physical exam screening tests such as making noise and looking for a head turn in a young child are inadequate in the setting of parental concern for hearing loss or delayed speech. No child is too young to have a hearing test.
Conventional Audiometry
For the cooperative child, typically 5 years of age or older, an audiometer can be used to measure ear-specific sensitivity to individual pure tones ranging from low tones at 256 Hertz to high tones at 8000 Hertz (Fig. 369-3). In most instances, pure tones can be heard at less than 20 to 25 decibels sound pressure level. In addition to pure tone thresholds, speech audiometry can be performed in these children. The speech reception threshold (SRT) refers to the intensity required to detect speech, as opposed to simple pure tones. The SRT is usually similar in value to the pure tone average, which is the average of the pure tone thresholds at 500, 1000, and 2000 Hertz. The speech recognition score refers to the child’s ability to recognize and repeat a standard set of phonetically balanced words presented at an intensity that can be heard comfortably, based on pure tone thresholds and SRT. These standard tests are invaluable in documenting a child’s baseline hearing level, determining whether the hearing level will affect the child’s development, and providing an objective means of assessing a child’s response to treatment for ear disease. For children who are too young to cooperate with these auditory techniques, behavioral testing represents a relatively inexpensive and noninvasive means of assessing a child’s hearing levels. Between the ages of 18 months and 5 years, children can be motivated to participate in activities that reflect whether a test stimulus was heard. Visual reinforcement audiometry involves testing the child in a sound-treated room with loudspeakers positioned at each side of the child. To maintain the child’s interest or to condition the child to respond when auditory stimuli are heard, a toy is typically activated near the speaker that elicited the response. By combining behavioral observation and visual reinforcement audiometry, the audiologist can usually obtain a reasonable impression of a child’s auditory capabilities.
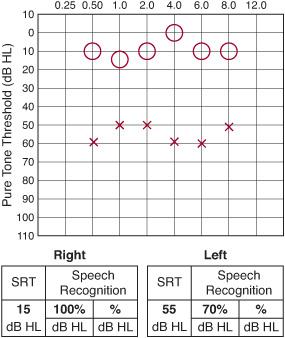
Figure 369-3. Conventional audiogram with left-sided hearing loss. O, right; X, left; SRT, speech reception threshold.
Evoked Auditory Brainstem Response and Otoacoustic Emission Testing
Auditory brainstem response (ABR) uses scalp electrodes placed on the skin to detect neural impulses following the delivery of various auditory stimuli to the ear. Computer-based averaging of neural activity allows for the calculation of “latencies” between distinct wave peaks. Evaluation of waveform morphology, the stimulus intensities required to elicit responses and the latencies of the wave peaks provide a qualitative assessment of the child’s auditory system.
Otoacoustic emission (OAE) testing is used to evaluate the peripheral auditory system. This method uses an extremely sensitive microphone that is placed in the external ear canal close to the tympanic membrane. Faint sounds generated by the outer hair cells of the cochlea are recorded. These sounds are generated either spontaneously or in response to auditory stimuli. The stimuli are referred to as transient evoked OAEs or distortion product OAEs. Because OAEs are believed to be generated by the outer hair cells of the cochlea, they may help identify or rule out the cochlear hair cells as the site of pathology in patients with sensorineural hearing loss (SNHL).
Immittance Audiometry/Tympanometry
Acoustic immittance is a generic term used to refer to either the opposition (impedance) or ease of entry (admittance) of acoustic energy into the middle ear transmission system. By means of a specialized earplug that seals off the external auditory canal, it is possible to measure the acoustic immittance of the ear. Contained within the earplug are a miniature speaker, air pump, and microphone. The ear normally absorbs sound energy through the tympanic membrane and middle ear structures. Impedance provides an indirect measure of this sound absorption function by measuring the reflected sound energy. The speaker delivers sound into the external auditory canal while the pressure is varied by the air pump. The microphone then detects the sound reflected back from the ear.
Several patterns of tympanograms are routinely encountered and reflect varying states of middle ear function (Fig. 369-4). The type A tympanogram is normal, with the curve peaking at approximately 0 cm H2O. The type B tympanogram is typically flat or shows only a very shallow peak. Such tympanograms are seen in cases of otitis media with effusion or ossicular fixation. A tympanic membrane perforation can also result in a flat tympanogram but is usually associated with an abnormally large canal volume; this volume is measured routinely during tympanometry. The type C tympanogram most commonly reflects a retracted tympanic membrane and shows a curve that peaks at pressures less than –150 cm H2O. Less reliably, tympanosclerosis and otosclerosis (caused by fixation of the stapes footplate) may be reflected in the type AS tympanogram. Last, the type AD tympanogram shows an abnormally high peak in an otherwise normal curve and is usually associated with ossicular discontinuity or an unusually mobile, atelectatic or atrophic tympanic membrane.
Vestibular (Balance) Testing
Several advances in the diagnosis of vestibular dysfunction have improved the diagnostic evaluation of children with dizziness. Electronystagmography (ENG) is extremely useful and is performed by applying electrodes around the eyes that detect the corneoretinal potential, which assesses eye movements. The function of the inner ear vestibular apparatus is evaluated by stimulating the inner ear with warm and cold water or air irrigation, which normally produces a characteristic nystagmus based on the vestibuloocular reflex. Cold irrigations in one ear normally cause a nystagmus in the opposite direction, whereas warm irrigations produce a nystagmus toward the ipsilateral ear. Quantification of the eye movements allows an objective measure of the vestibular function of each inner ear and can provide data for discriminating between peripheral and central vestibular dysfunction. More recent improvements in ENG testing include the use of video-infrared ENG systems, referred to as VNG. These systems involve video cameras mounted inside a pair of goggles that “lock onto” the retina by means of a computer-controlled mechanism.
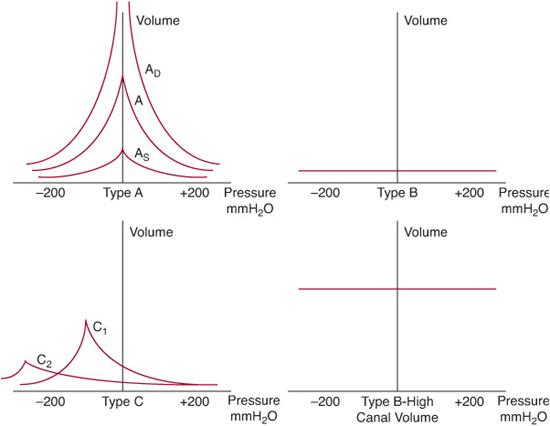
Figure 369-4. Illustration of various types of tympanograms. A, normal tympanogram, maximal peak (compliance) seen at 0 mm H2O; AD, deep (hypermobile tympanic membrane as seen with atelectasis or ossicular discontinuity); AS, shallow (stiff tympanic membrane as seen with tympanosclerosis or otosclerosis); B, nonmobile tympanic membrane, normal canal volume (a middle ear effusion); type B high canal volume, nonmobile tympanic membrane has no pressure differential across it (tympanostomy tube or perforation); C1, mild negative middle ear pressure (eustachian tube dysfunction); C2, negative middle ear pressure below –200 mm H2O (usually a middle ear effusion).
Computerized platform posturography also represents a significant advance in vestibular testing. By using a platform that the test subject stands on, the child’s center of gravity can be recorded both statistically as well as in response to movement of the platform. Data from such tests provide insight into the child’s overall balance function, be it centrally or peripherally controlled. Certain patterns of body movements or failures to compensate for platform movement can indicate cerebellar dysfunction.
In addition, rotary chair testing allows for the audiologist to provide data on the function of the vestibular system, even when there is minimal patient cooperation. The child sits with or without the parent in a slowly rotating chair while eye measurements are recorded.
 RADIOGRAPHIC EVALUATION
RADIOGRAPHIC EVALUATION
Computed tomography (CT) and magnetic resonance imaging (MRI) are currently the standard of care in evaluating children with otologic disease.3 Optimal axial and coronal CT images of the ear using 1-millimeter cuts through the temporal bone identify minute pathology that can be significant in the middle ear, mastoid, and inner ear regions. Bone window images typically provide the greatest information regarding the external, middle, and inner ear regions. In the external and middle ear, CT can demonstrate opacification of the air spaces, erosion of bony structures or ossicles, atretic plates of the external canal, and other middle ear congenital abnormalities. In the inner ear, CT can clearly show the auditory and vestibular structures of the inner ear labyrinth, as well as the internal auditory canal that transmits the facial, cochlear, and vestibular nerves. CT also readily demonstrates aplasias and other malformations of the inner ear. Notably, an enlarged vestibular aqueduct (EVA) is best demonstrated by CT.
Gadolinium-enhanced MRI provides an excellent means for assessing the soft tissue structures of the ear. One-millimeter cuts through the temporal bone accurately visualize critical neural structures, including the facial, cochlear, and vestibular nerves. With the use of gadolinium, these structures can also be evaluated for signs of inflammation or neo-plastic involvement. Enlargements of the endolymphatic sac are best seen on MRI studies.4
DISORDERS OF THE EAR
CONGENITAL AND ACQUIRED HEARING LOSS
 EPIDEMIOLOGY
EPIDEMIOLOGY
Approximately 1 in 1500 children has a severe to profound hearing loss at birth or in early childhood. This relatively high incidence, the high likelihood of significant negative developmental impact in children with hearing loss, and the significant technological capabilities to habilitate children with hearing loss have lead to most developed countries instituting universal newborn hearing screening protocols. These protocols are designed to identify all children with hearing loss at an early age so that intervention can be provided during the critical early periods of speech and language development.5,6 The most common causes of acquired hearing loss in children is a conductive hearing loss that results from abnormalities of the middle ear (especially otitis media and ossicular abnormalities), whereas congenital hearing loss is often a result of sensorineural deficits.
 PATHOPHYSIOLOGY AND GENETICS
PATHOPHYSIOLOGY AND GENETICS
eTable 369.1  lists common causes of congenital hearing loss. Various series estimate that upward of 50% of these cases are genetic in origin (eFig. 369.1
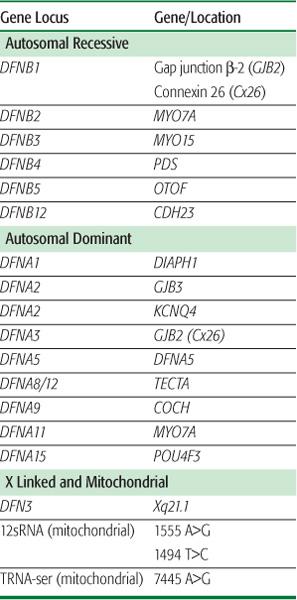
lists common causes of congenital hearing loss. Various series estimate that upward of 50% of these cases are genetic in origin (eFig. 369.1  ). Of these patients, approximately 70% have isolated hearing loss as the only phenotypic manifestation (ie, nonsyndromic hereditary hearing impairment). The remaining have hearing loss in conjunction with other abnormalities (ie, syndromic hearing impairment associated with Pendred, Usher, Wardenburg, Brachio-oto-renal, and other syndromes). In patients with nonsyndromic hereditary hearing impairment, the vast majority (∼80%) display an autosomal-recessive mode of transmission. Another 15% are estimated to have an autosomal-dominant mode of inheritance, with the remainder being X linked or mitochondrial in nature. The known genetic disorders associated with hearing loss are found in Table 369-1.
). Of these patients, approximately 70% have isolated hearing loss as the only phenotypic manifestation (ie, nonsyndromic hereditary hearing impairment). The remaining have hearing loss in conjunction with other abnormalities (ie, syndromic hearing impairment associated with Pendred, Usher, Wardenburg, Brachio-oto-renal, and other syndromes). In patients with nonsyndromic hereditary hearing impairment, the vast majority (∼80%) display an autosomal-recessive mode of transmission. Another 15% are estimated to have an autosomal-dominant mode of inheritance, with the remainder being X linked or mitochondrial in nature. The known genetic disorders associated with hearing loss are found in Table 369-1.
Studies of large kindreds with hearing loss have identified multiple genetic loci associated with nonsyndromic hearing impairment. To date, 21 autosomal-dominant (DFNA) genes, 22 autosomal-recessive (DFNB) genes, and 1 X-linked (DFN) gene have been identified. An illustrative example of the clinical relevance of these genetic discoveries can be seen with the gene GJB2 (gap junction ?-2 protein), which is also known as connexin 26 (CX 26).7 Studies have identified more than 100 different mutations in GJB2 as a cause for hearing loss. The most common mutation is 35delG, which accounts for about one third of all mutations. Routine lab testing is now used to identify CX 26 mutations, which subsequently helps predict future hearing deterioration and success with cochlear implantation. Mutations in genes such as SLC26A4, 12SrRNA, MYO7A, OTOF, and CDH23 are also believed to account for a significant proportion of hearing loss in the general population.
A history of maternal “TORCH” infections (Toxoplasmosis, Other agents such as Coxsackie virus and Listeria, Rubella, Cytomegalovirus [CMV], Herpes simplex) during pregnancy can indicate a likely etiology for congenital hearing loss in a child. Rubella, for example, is particularly associated with cochleosaccular dysplasia and congenital deafness. Prenatal CMV infection may account for up to 40% of all children with sensorineural hearing loss (SNHL) (see Chapter 310). SNHL is seen in 5% to 10% of children with asymptomatic CMV infection and in more than 50% of children with symptomatic CMV infection. A history of maternal drug use during pregnancy may also be important. For example, isotretinoin causes congenital hearing loss with associated malformations of the cochlea. Substances such as alcohol, cocaine, and other “recreational” drugs may also cause congenital hearing loss. Perinatal factors such as prematurity, low birth weight, low Apgar scores, and the need for neonatal intensive care unit (NICU) admission have all been correlated with SNHL. Complicated delivery with infant anoxia or severe dystocia requiring forceps delivery may provide insight into the cause of congenital hearing loss. Traumatic deliveries can cause mastoid and middle ear damage that result in conductive hearing loss and/or SNHL.
Table 369-1. Common Genes Associated with Nonsyndromic Sensorineural Hearing Loss
During the very early postnatal period, routine screening of infants for problems such as hypothyroidism or phenylketonuria is now commonplace. Therefore, the likelihood of missing these possible etiologies of hearing loss is minimal. Other risk factors for hearing loss at this time include hyperbilirubinemia (typically > 17.0 mg/dL), metabolic defects, and a wide range of hereditary congenital processes that manifest with hearing loss.
 DIAGNOSIS
DIAGNOSIS
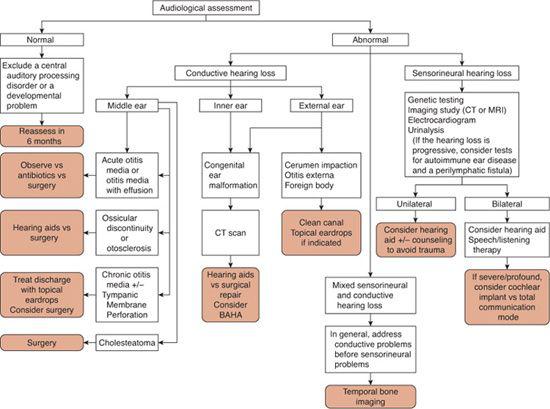
The physical examination of the ear in infants should focus on identifying features that might suggest associated inner ear anomalies. For example, stenosis or atresia of the external auditory canal associated with preauricular pits or skin tags can suggest branchio-oto-renal syndrome, a fairly common congenital deafness syndrome associated with malformation of the cochlea. Early audiometric testing is essential in suspected congenital hearing loss. As discussed previously, accurate audiometric results can be obtained on any age child through behavioral testing and/or auditory brainstem response (ABR) and otoacoustic emission (OAE) testing. Figure 369-5 is an algorithm for the diagnostic assessment and treatment of patients with either conductive or sensorineural hearing loss.
MANAGEMENT OF CHILDREN WITH CONFIRMED HEARING LOSS
The management options for children with confirmed hearing loss have changed dramatically over the past decade. Figure 369-5 provides an algorithm for the evaluation and management of children with suspected hearing loss, and eFigure 369.2  provides an approach to the management of children with hearing loss detected at infant screening. Once hearing loss is confirmed using an age-appropriate audiologic modality described earlier in this chapter (behavioral audiometry, auditory brainstem response [ABR], otoacoustic emissions [OAEs]), evaluation next is focused upon determining if the hearing loss is due to a sensorineural hearing loss (SNHL) or conductive hearing loss (CHL).
provides an approach to the management of children with hearing loss detected at infant screening. Once hearing loss is confirmed using an age-appropriate audiologic modality described earlier in this chapter (behavioral audiometry, auditory brainstem response [ABR], otoacoustic emissions [OAEs]), evaluation next is focused upon determining if the hearing loss is due to a sensorineural hearing loss (SNHL) or conductive hearing loss (CHL).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


