Drug
Dose
Response rate (%)
Cisplatin
50–100 mg/m2 every 3 weekly
18–27
Doxorubicin
50–60 mg/m2 every 3 weekly
22–32
Paclitaxel
175–250 mg/m2 every 3 weekly
36
In order to improve the response rates, combination chemotherapy was introduced. Initially two drug combinations and later three drug combinations were used (Table 28.2).
Table 28.2
Response rates to combination chemotherapy
Combination | Number of patients | CR + PR% | Ref |
|---|---|---|---|
Cyclophosphamide + doxorubicin | 26 | 31 | [6] |
Doxorubicin + cisplatin | 30 | 60 | [7] |
Cyclophosphamide + doxorubicin + cisplatin | 87 | 45 | [8] |
Paclitaxel + doxorubicin + cisplatin | 133 | 57 | [9] |
Paclitaxel + carboplatin | 1300 | 51 | [10] |
GOG 122 trial results were published in 2006, and this compared whole-abdominal irradiation (WAI) versus doxorubicin and cisplatin (AP) chemotherapy in advanced endometrial carcinoma [9]. At 5 years, adjusting for stage, 55 % of AP patients were predicted to be alive compared with 42 % of WAI patients. Significant side effects of AP therapy are hematologic, which includes grade 3/4 neutropenia (55 %) and non-hematologic, namely, grade 3/4 alopecia (72 %) and nausea/vomiting (36 %). In the GOG 177 trial, addition of paclitaxel improved objective response (57 % vs. 34 %; P < 0.01), PFS (median, 8.3 vs. 5.3 months; P < 0.01), and OS (median, 15.3 vs. 12.3 months; P = 0.037) [11]. Use of growth factor support ensured that febrile neutropenia was just 3 % in the TAP arm (doxorubicin, cisplatin, and paclitaxel) compared to AP (cisplatin + doxorubicin). Neurologic toxicity was worse for those receiving TAP, with 12 % grade 3 and 27 % grade 2 peripheral neuropathy, compared with 1 % and 4 %, respectively, in those receiving AP. As endometrial carcinoma is a disease of the elderly with multiple comorbidities, intensification of chemotherapy led to an increased but tolerable rise in toxicities. From a chemotherapy perspective, cisplatin is highly emetic, and drug delivery requires close monitoring of hydration to prevent complications. As carboplatin was being substituted in many other solid malignancies, the same approach was tested in GOG 209. In chemotherapy, naive women with stage III, IV, or recurrent disease doxorubicin, cisplatin, and paclitaxel (TAP) were compared with carboplatin and paclitaxel (TC). In this non-inferiority trial, the PFS (median PFS of TAP vs. TC: 13.5 vs. 13.3 months) and OS (40.3 vs. 36.5 months) were similar. The toxicity profile favored TC with less sensory neuropathy (sensory neuropathy >grade 1: 26 % vs. 19 %, p < 0.01) [10] in TC regimen; chemo schedule is as carboplatin AUC = 6 mg/mL/min IV + paclitaxel 175 mg/m2 IV repeated every 3 weeks. TAP schedule is as doxorubicin [45 mg/m2 on day 1], cisplatin 50 mg/m2 on day 1 plus paclitaxel 160 mg/m2 over 3 h on day 2 every 3 weeks with growth factor support.
In a recurrent setting, selective proliferation of chemoresistant cells occurs. Tumor cells may develop resistance to paclitaxel by overexpression of the multidrug-resistance gene (MDR-1), which encodes P-glycoprotein (P-gp), an efflux pump that prevents accumulation of a variety of natural product-based chemotherapeutic agents. Five-year survival rate for patients with advanced/recurrent measurable disease is <10 %, and for those with stage III disease, it is typically around 50–60 %. As use of chemotherapy is increasing in the adjuvant setting, dose-limiting toxicities like cardiotoxicity with doxorubicin and sensory neuropathy with paclitaxel have to be taken into consideration. In a GOG analysis of approximately 1200 patients with recurrent disease, factors independently associated with longer survival included white/Hispanic race, better performance status, stage III disease, no prior radiation therapy, and endometrioid tumor histology. The best response rates with chemotherapy in a recurrent setting were in the range of 9–13 % [12]. In a Phase 2 clinical trial, epothilone B analogue ixabepilone 40 mg/m2 as a 3-h infusion on day 1 of a 21-day cycle was recently reported to have a 12 % response rate in an extensively pretreated population [13].
Targeted Therapy
In endometrial cancer, there is a subset of hormonally sensitive disease. In high-grade, extensive, and recurrent tumors, chemotherapy has limited, short-lasting benefit. Genetic basis of carcinogenesis is a multistep process progressing through initiation, promotion, and invasion. Mutations in genes like K ras, P 53, and PTEN have been described in endometrial carcinoma. In endometrial cancer, the mutation of genes associated with cancer initiation varies with clinical characteristics (type I or II), tissue differentiation, and histological type (Table 28.3) [14]. Mutations accumulate when DNA repair mechanisms are defective. hMSH2 and hMLH1 alterations in endometrial carcinomas are associated with Lynch syndrome. Epigenetic changes like hypermethylation of the hMLH1 promoter reduces the ability to repair mismatches during DNA replication, leading to mutations of phosphatase and tensin homolog (PTEN) and subsequent generation of endometrioid adenocarcinoma [15]. As this is a stepwise progression, there is scope of intervention at any of these steps to arrest cancer growth.
Table 28.3
Genetic mutations in endometrioid and non-endometrioid cancers
Alteration | Endometrioid (%) | Non-endometrioid (%) |
|---|---|---|
PTEN mutation | 30–50 | 0–11 |
PIK3CA mutation | 30–40 | 20 |
KRAS mutation | 10–30 | 0–10 |
EGFR overexpression | 46 | 34 |
HER-2 overexpression | 3–10 | 32 |
p53 mutation | 20 | 90 |
Microsatellite instability | 15–25 | 0–5 |
NCCN guidelines have recommended the use of two molecules, temsirolimus and bevacizumab, which are discussed in detail. Trials are ongoing for multiple other drugs, in combination and sequentially, but they have not come to the level of recommendation [14]. Although there are a large number of studies about the molecular alterations in endometrial carcinomas, the clinically relevant ones with druggable targets and their response rates are given in Table 28.4
Table 28.4
Targeted therapies and response rates in endometrial cancer
Drug | Response rate (%) |
|---|---|
Angiogenesis inhibitors | |
Bevacizumab | 13.5 |
Aflibercept | 6.8 |
Thalidomide | 12.5 |
EGFR inhibitors | |
Gefitinib | 3.4 |
Erlotinib | 4.3 |
HER2 neu inhibitors | |
Trastuzumab | 0.0 |
mTOR inhibitors | |
Temsirolimus | 26.0 |
Ridaforolimus | 28.9 (CBR) |
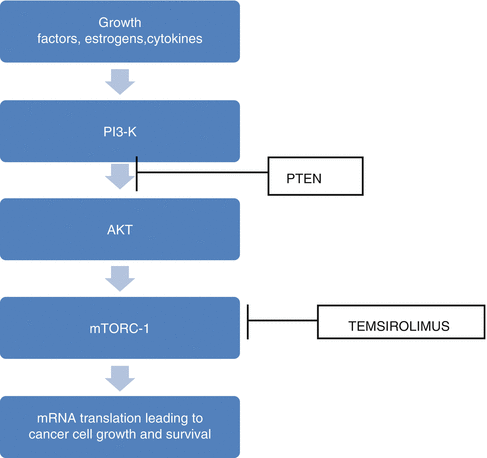
Temsirolimus
PI3K/AKT pathway plays a central role in cell survival, growth, and avoidance of apoptosis. Stimulation of the PI3K/AKT pathway occurs through the activity of receptors including epidermal growth factor receptor (EGFR), insulin-like growth factor I receptor (IGFIR), etc. Constitutive activation of the PI3K/AKT pathway in endometrial cancer occurs most commonly through loss of PTEN (phosphatase and tensin homolog, a tumor suppressor gene). The mammalian target of rapamycin (mTOR), a serine/threonine kinase, is a critical downstream target of the PI3K/AKT pathway (Fig. 28.1). mTOR upregulation through AKT leads to subsequent activation of the protein S6 kinase (pS6K) which regulates protein translation and cell cycle progression [16].