Background
Optimal care for women with endometrial cancers often involves transfer of care from diagnosing physicians (eg, obstetrician-gynecologists) to treating physicians (eg, gynecologic oncologists.) It is critical to determine the effect of time to treatment on cancer outcomes to set best practices guidelines for referral processes.
Objective
We sought to determine the impact of time from diagnosis of endometrial cancer to surgical treatment on mortality and to characterize those patients who may be at highest risk for worsened survival related to surgical timing.
Study Design
The National Cancer Database was queried for incident endometrial cancers in adults from 2003 through 2012. Cancers were classified as low risk (grade 1 or 2 endometrioid histologies) or high risk (nonendometrioid and grade 3 endometrioid histologies) and analyzed separately. Demographic, clinicopathologic, and health system factors were collected. Unadjusted and adjusted hazard ratios for mortality were calculated by interval between diagnosis and surgery. Linear regression of patient and health care system characteristics was performed on diagnosis-to-surgery interval.
Results
For low-risk cancers (N = 140,078), surgery in the first and second weeks after diagnosis was independently associated with mortality risk (hazard ratio, 1.4; 95% confidence interval, 1.3–1.5; and hazard ratio, 1.1; 95% confidence interval, 1.0–1.2, respectively). The 30-day postoperative mortality was significantly higher among patients undergoing surgery in the first or second week postdiagnosis, compared to patients treated in the third or fourth week postdiagnosis (0.7% vs 0.4%; P < .001). Mortality risk was also significantly higher than baseline when time between diagnosis and surgery was >8 weeks. Independent associations with added time to surgery of at least 1 week were seen with black race (1.1 weeks; 95% confidence interval, 0.9–1.4), uninsurance (1.3 weeks; 95% confidence interval, 1.1–1.5), Medicaid insurance (1.7 weeks; 95% confidence interval, 1.5–1.9), and Charlson-Deyo comorbidity score >1 (1.0 weeks; 95% confidence interval, 0.8–1.2). For high-risk cancers (N = 68,360), surgery in the first and second weeks after diagnosis was independently associated with mortality risk (hazard ratio, 1.5; 95% confidence interval, 1.3–1.6; and hazard ratio, 1.2; 95% confidence interval, 1.1–1.2, respectively). The 30-day postoperative mortality was significantly higher among patients undergoing surgery in the first or second week postdiagnosis, compared to patients treated in the third or fourth week postdiagnosis (2.5% vs 1.0%; P < .001). Surgery after the third week postdiagnosis was not associated with a statistically significant increase in the adjusted risk of mortality. Independent associations with added time to surgery of at least 1 week were seen with uninsurance (1.4 weeks; 95% confidence interval, 0.9–1.9) and Medicaid insurance (1.4 weeks; 95% confidence interval, 1.1–1.7).
Conclusion
Surgery in the first 2 weeks after diagnosis of endometrial cancer was associated with worsened survival associated with elevated perioperative mortality and treatment in low-volume hospitals. Delay in surgical treatment was a risk factor for mortality in low-risk cancers only and was likely associated with poor access to specialty care. We suggest that the target interval between diagnosis and treatment of endometrial cancers be ≤8 weeks; however, referral to an experienced surgeon and adequate preoperative optimization should be prioritized over expedited surgery.
Introduction
Delay between diagnosis and surgical treatment of endometrial cancer may result in worsened overall survival, potentially as a consequence of disease progression or difficulty accessing care. A relationship between surgical delay and survival disadvantage has been demonstrated in breast, rectal, and bladder cancers; this relationship does not clearly exist for esophageal, gastric, renal cell, or cervical cancers. For endometrial cancer, findings to date have been mixed. Early work suggested that time to definitive treatment did not correlate with disease stage or survival ; however, these studies were limited by small sample sizes, mixed tumor histologies, and a focus on time from onset of abnormal uterine bleeding rather than from definite diagnosis of malignancy.
Recently, 3 studies readdressed this issue with larger sample populations. A 2013 report of >9000 patients in Canada associated longer wait times with lower overall survival at 5 years. Although this study was criticized for including high-risk histologies, a subsequent subset analysis of >3000 patients included only endometrioid cancers undergoing simple hysterectomy and excluded patients receiving chemotherapy or radiation. A survival disadvantage was confirmed for women undergoing surgery <2 weeks after diagnosis or waiting >12 weeks for hysterectomy. In contrast, a study of 435 patients in California with grade 1-2 endometrioid-type endometrial cancer did not show an impact of wait time on overall survival, but was criticized for being underpowered to do so adequately. A third study using the National Cancer Database (NCDB) associated a diagnosis-to-surgery interval of ≥6 weeks with worsened outcomes; however this study analyzed a single time point and combined high- and low-risk histologies.
Based on available data, we hypothesized that delayed or early surgical intervention may be associated with poor outcomes. Furthermore, the relationship between surgical interval and outcomes is likely to be different for low- and high-risk cancers. We therefore analyzed a large patient sample drawn from the NCDB to determine whether and when time from diagnosis of endometrial cancer to surgical treatment affects mortality and to characterize those patients who may be at highest risk for worsened survival related to timing of surgery.
Materials and Methods
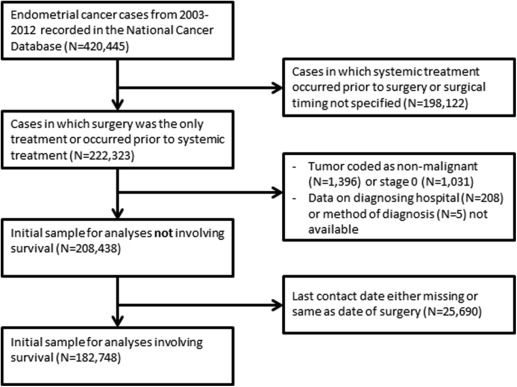
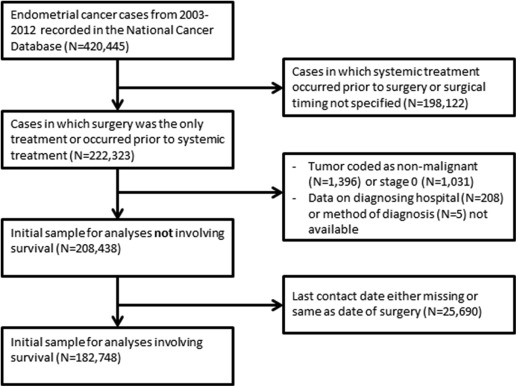
The NCDB, maintained by the American College of Surgeons and the American Cancer Society, captures approximately 70% of cancer cases in the United States from 1500 Commission on Cancer (CoC)-accredited institutions nationwide. The NCDB was queried for cases of endometrial cancer from 2003 through 2012. Cases included in the uterine corpus database with epithelial histologies were considered to be of endometrial origin. Low-risk (grade 1 and grade 2 endometrioid histologies) and high-risk (grade 3 endometrioid and all other epithelial histologies) tumors were analyzed separately. Uterine carcinosarcoma was included in the high-risk epithelial group, as this tumor likely originates from a dedifferentiated carcinoma. There were 420,445 patients in the initial sample. We limited our analysis to cases for which there was evidence that surgery was the only modality pursued, or occurred prior to any hormonal therapy, radiation, or chemotherapy. We excluded those for whom time between diagnosis and surgery was unavailable or diagnosis was made at the time of surgery. In all, 222,323 cases met initial inclusion criteria. We then excluded cases for which the tumor was coded as nonmalignant (N = 1396) or stage 0 (N = 1031), or for which diagnostic confirmation (N = 5) or hospital identifier (N = 208) were invalid. For analyses not including survival time, the sample consisted of 208,438 patient-level observations. For analyses involving survival, we further excluded cases for which the last contact date was missing or equaled the treatment date, leaving 182,748 patients ( Figure 1 ).
Variables
Covariates included patient, facility, and geographic area characteristics. Patient characteristics included age (<45, 45–54, 55–64, 65–74, 75–84, ≥85 years), race (white, black, American Indian, Asian/Pacific Islander, other, and unknown), ethnicity (Hispanic vs not), primary payer (not insured, private, Medicaid, Medicare, other government), stage (1, 2, 3, or 4 based on the higher of pathologic and clinical stages; or unknown), grade (1, 2, 3, or unknown), receipt of systemic (chemotherapy or hormonal therapy) or radiation therapy within 60 days after surgery, treatment and/or diagnosis in the reporting facility, Charlson-Deyo comorbidity score (0, 1, 2+, excluding cancer), performance of lymphadenectomy, and year of diagnosis. Facility characteristics included type (community cancer program, comprehensive community cancer program, academic/research program) and quartile of annual endometrial cancer cases (calculated prior to sample exclusions). Geographic area characteristics included facility’s census region, quartile of straight-line distance from patient’s residential ZIP code to facility, metropolitan location of patient’s ZIP code (yes/no), and quartile of patient’s ZIP code–level median household income (based on 2000 US Census). Lymphadenectomy was collected as a proxy for gynecologic oncologist involvement in surgical treatment and was not expected to be independently associated with survival in these cases, although controversy exists on this point. The location of lymph nodes removed (eg, pelvic vs paraaortic) was not documented in the NCDB. The interval from diagnosis to surgery was coded as the week postdiagnosis during which surgery occurred; for example, surgery on days 1-7 postdiagnosis was coded as occurring during week 1, and on days 8-14 as week 2. The date of diagnosis was originally recorded as either the date a confirmatory test (eg, biopsy) was performed or the date that a clinical diagnosis was documented, whichever came first.
Statistical analysis
The unit of analysis was the individual patient. Covariate and outcome values were compared across the 2 tumor categories (high or low risk) using χ 2 tests for categorical variables and nonparametric equality of medians tests for continuous variables. To assess the association between surgical delay and postsurgical time to death or censoring separately for high- and low-risk cancers, the Kaplan-Meier method was used to calculate crude 5-year survival for each week of delay and Cox proportional hazard models were used to estimate crude and adjusted hazard ratios (HR). Linear regression was used to identify independent predictors of time between cancer diagnosis and definitive surgery. To account for the possible role of outliers in the distribution of time from diagnosis to surgery, we also estimated quasimaximum likelihood Poisson models. We report only the linear regression results because the Poisson results are qualitatively identical. In all models, SE were adjusted to account for the clustering of patients within centers. All analyses used software (Stata, Version 14.2; Stata Corp, College Station, TX). Two-tailed P < .05 was considered statistically significant. As the NCDB is a deidentified database, this study was exempted from institutional review board review.
Materials and Methods
The NCDB, maintained by the American College of Surgeons and the American Cancer Society, captures approximately 70% of cancer cases in the United States from 1500 Commission on Cancer (CoC)-accredited institutions nationwide. The NCDB was queried for cases of endometrial cancer from 2003 through 2012. Cases included in the uterine corpus database with epithelial histologies were considered to be of endometrial origin. Low-risk (grade 1 and grade 2 endometrioid histologies) and high-risk (grade 3 endometrioid and all other epithelial histologies) tumors were analyzed separately. Uterine carcinosarcoma was included in the high-risk epithelial group, as this tumor likely originates from a dedifferentiated carcinoma. There were 420,445 patients in the initial sample. We limited our analysis to cases for which there was evidence that surgery was the only modality pursued, or occurred prior to any hormonal therapy, radiation, or chemotherapy. We excluded those for whom time between diagnosis and surgery was unavailable or diagnosis was made at the time of surgery. In all, 222,323 cases met initial inclusion criteria. We then excluded cases for which the tumor was coded as nonmalignant (N = 1396) or stage 0 (N = 1031), or for which diagnostic confirmation (N = 5) or hospital identifier (N = 208) were invalid. For analyses not including survival time, the sample consisted of 208,438 patient-level observations. For analyses involving survival, we further excluded cases for which the last contact date was missing or equaled the treatment date, leaving 182,748 patients ( Figure 1 ).
Variables
Covariates included patient, facility, and geographic area characteristics. Patient characteristics included age (<45, 45–54, 55–64, 65–74, 75–84, ≥85 years), race (white, black, American Indian, Asian/Pacific Islander, other, and unknown), ethnicity (Hispanic vs not), primary payer (not insured, private, Medicaid, Medicare, other government), stage (1, 2, 3, or 4 based on the higher of pathologic and clinical stages; or unknown), grade (1, 2, 3, or unknown), receipt of systemic (chemotherapy or hormonal therapy) or radiation therapy within 60 days after surgery, treatment and/or diagnosis in the reporting facility, Charlson-Deyo comorbidity score (0, 1, 2+, excluding cancer), performance of lymphadenectomy, and year of diagnosis. Facility characteristics included type (community cancer program, comprehensive community cancer program, academic/research program) and quartile of annual endometrial cancer cases (calculated prior to sample exclusions). Geographic area characteristics included facility’s census region, quartile of straight-line distance from patient’s residential ZIP code to facility, metropolitan location of patient’s ZIP code (yes/no), and quartile of patient’s ZIP code–level median household income (based on 2000 US Census). Lymphadenectomy was collected as a proxy for gynecologic oncologist involvement in surgical treatment and was not expected to be independently associated with survival in these cases, although controversy exists on this point. The location of lymph nodes removed (eg, pelvic vs paraaortic) was not documented in the NCDB. The interval from diagnosis to surgery was coded as the week postdiagnosis during which surgery occurred; for example, surgery on days 1-7 postdiagnosis was coded as occurring during week 1, and on days 8-14 as week 2. The date of diagnosis was originally recorded as either the date a confirmatory test (eg, biopsy) was performed or the date that a clinical diagnosis was documented, whichever came first.
Statistical analysis
The unit of analysis was the individual patient. Covariate and outcome values were compared across the 2 tumor categories (high or low risk) using χ 2 tests for categorical variables and nonparametric equality of medians tests for continuous variables. To assess the association between surgical delay and postsurgical time to death or censoring separately for high- and low-risk cancers, the Kaplan-Meier method was used to calculate crude 5-year survival for each week of delay and Cox proportional hazard models were used to estimate crude and adjusted hazard ratios (HR). Linear regression was used to identify independent predictors of time between cancer diagnosis and definitive surgery. To account for the possible role of outliers in the distribution of time from diagnosis to surgery, we also estimated quasimaximum likelihood Poisson models. We report only the linear regression results because the Poisson results are qualitatively identical. In all models, SE were adjusted to account for the clustering of patients within centers. All analyses used software (Stata, Version 14.2; Stata Corp, College Station, TX). Two-tailed P < .05 was considered statistically significant. As the NCDB is a deidentified database, this study was exempted from institutional review board review.
Results
In all, 140,078 low-risk and 68,360 high-risk endometrial cancers were included in the descriptive analysis ( Table 1 ). As expected, low-risk cancers occurred comparatively more frequently in younger women and less frequently in black women. Women with low-risk cancers were more likely to be diagnosed with stage I or II disease than women with high-risk cancers. Women with high-risk cancers were more likely to have Medicare insurance and less likely to be privately insured compared to women with low-risk cancers. Hispanic ethnicity, comorbidity score, and annual hospital case volume were not significantly different between low- and high-risk cancer cases.
| Overall (N = 208,438) | Low risk (N = 140,078) | High risk (N = 68,360) | P value | |
|---|---|---|---|---|
| Age at diagnosis, y | <.001 | |||
| Mean (SD) | 62.86 (11.63) | 61.82 (11.59) | 64.97 (11.44) | |
| Age category, y, N (%) | <.001 | |||
| <45 | 11,926 (5.7%) | 9138 (6.5%) | 2788 (4.1%) | |
| 45–54 | 34,529 (17%) | 25,891 (18%) | 8638 (13%) | |
| 55–64 | 72,751 (35%) | 50,688 (36%) | 22,063 (32%) | |
| 65–74 | 53,645 (26%) | 33,463 (24%) | 20,182 (30%) | |
| 75–84 | 28,857 (14%) | 17,018 (12%) | 11,839 (17%) | |
| ≥85 | 6730 (3.2%) | 3880 (2.8%) | 2850 (4.2%) | |
| Patient race, N (%) | <.001 | |||
| White | 179,598 (86%) | 123,913 (88%) | 55,685 (81%) | |
| Black | 18,880 (9.1%) | 9332 (6.7%) | 9548 (14%) | |
| American Indian | 584 (0.3%) | 414 (0.3%) | 170 (0.2%) | |
| Asian/Pacific Islander | 4972 (2.4%) | 3369 (2.4%) | 1603 (2.3%) | |
| Other | 1511 (0.7%) | 1024 (0.7%) | 487 (0.7%) | |
| Unknown | 2893 (1.4%) | 2026 (1.4%) | 867 (1.3%) | |
| Hispanic ethnicity, N (%) | .032 | |||
| No | 181,666 (87%) | 122,039 (87%) | 59,627 (87%) | |
| Yes | 10,023 (4.8%) | 6659 (4.8%) | 3364 (4.9%) | |
| Unknown | 16,749 (8.0%) | 11,380 (8.1%) | 5369 (7.9%) | |
| Insurance coverage, N (%) | <.001 | |||
| Not insured | 7235 (3.5%) | 4921 (3.5%) | 2314 (3.4%) | |
| Private insurance | 102,789 (49%) | 73,658 (53%) | 29,131 (43%) | |
| Medicaid | 9095 (4.4%) | 6020 (4.3%) | 3075 (4.5%) | |
| Medicare | 83,771 (40%) | 51,913 (37%) | 31,858 (47%) | |
| Other government | 1783 (0.9%) | 1258 (0.9%) | 525 (0.8%) | |
| Unknown insurance | 3765 (1.8%) | 2308 (1.6%) | 1457 (2.1%) | |
| Tumor stage, N (%) | <.001 | |||
| 1 | 145,628 (70%) | 107,468 (77%) | 38,160 (56%) | |
| 2 | 15,196 (7.3%) | 9608 (6.9%) | 5588 (8.2%) | |
| 3 | 26,777 (13%) | 13,623 (9.7%) | 13,154 (19%) | |
| 4 | 8953 (4.3%) | 2685 (1.9%) | 6268 (9.2%) | |
| Unknown | 11,884 (5.7%) | 6694 (4.8%) | 5190 (7.6%) | |
| Charlson-Deyo score, a N (%) | .40 | |||
| 0 | 155,646 (75%) | 104,509 (75%) | 51,137 (75%) | |
| 1 | 43,068 (21%) | 29,057 (21%) | 14,011 (20%) | |
| 2+ | 9724 (4.7%) | 6512 (4.6%) | 3212 (4.7%) | |
| Facility type, N (%) | <.001 | |||
| Community cancer program | 12,048 (5.8%) | 7860 (5.6%) | 4188 (6.1%) | |
| Comprehensive community cancer program | 107,244 (51%) | 72,399 (52%) | 34,845 (51%) | |
| Academic/research program | 88,747 (43%) | 59,524 (42%) | 29,223 (43%) | |
| Other | 399 (0.2%) | 295 (0.2%) | 104 (0.2%) | |
| Annual hospital volume quartile, N (%) | <.001 | |||
| 1 Lowest | 40,735 (20%) | 26,767 (19%) | 13,968 (20%) | |
| 2 | 44,106 (21%) | 29,409 (21%) | 14,697 (21%) | |
| 3 | 56,861 (27%) | 38,434 (27%) | 18,427 (27%) | |
| 4 Highest | 66,736 (32%) | 45,468 (32%) | 21,268 (31%) | |
| Distance traveled to recording institution, miles b | ||||
| Mean (SD) | 30.52 (89.29) | 30.10 (86.67) | 31.40 (94.43) | .002 |
| Median (IQR) | 11.70 (25.40) | 11.80 (25.10) | 11.40 (25.80) | <.001 |
| Metro or adjacent to metro, N (%) b | <.001 | |||
| No | 22,094 (11%) | 14,583 (10%) | 7511 (11%) | |
| Yes | 186,344 (89%) | 125,495 (90%) | 60,849 (89%) | |
| Median income quartile, N (%) b | <.001 | |||
| 1 Lowest | 30,131 (15%) | 18,953 (14%) | 11,178 (17%) | |
| 2 | 42,060 (21%) | 28,107 (21%) | 13,953 (21%) | |
| 3 | 56,284 (28%) | 38,207 (28%) | 18,077 (27%) | |
| 4 Highest | 74,498 (37%) | 51,208 (38%) | 23,290 (35%) | |
| Household education, quartile, N (%) b | <.001 | |||
| 1 Lowest | 31,766 (16%) | 20,125 (15%) | 11,641 (18%) | |
| 2 | 49,073 (24%) | 32,453 (24%) | 16,620 (25%) | |
| 3 | 60,232 (30%) | 41,311 (30%) | 18,921 (28%) | |
| 4 Highest | 61,932 (31%) | 42,603 (31%) | 19,329 (29%) | |
| Lymphadenectomy, N (%) | <.001 | |||
| No | 54,719 (26%) | 38,477 (27%) | 16,242 (24%) | |
| Yes | 153,719 (74%) | 101,601 (73%) | 52,118 (76%) | |
| Adjuvant systemic therapy, N (%) | <.001 | |||
| No | 182,641 (88%) | 130,821 (93%) | 51,820 (76%) | |
| Yes | 25,797 (12%) | 9257 (6.6%) | 16,540 (24%) | |
| Adjuvant radiation therapy, N (%) | <.001 | |||
| No | 178,496 (86%) | 120,644 (86%) | 57,852 (85%) | |
| Yes | 29,942 (14%) | 19,434 (14%) | 10,508 (15%) | |
a Excludes cancer as comorbidity
Survival analyses for low-risk cancers
For patients with low-risk cancers, median survival time was 47.6 months (interquartile range 25.8-73.6), and 14.3% of cases were censored. Five-year crude survival was highest when surgery was performed in the third week after diagnosis, with a linear decline in survival thereafter ( Table 2 ; Supplemental Figure 1 ). Relative to patients who underwent surgery in the third week after diagnosis, the 11.7% of patients undergoing surgery during the first and second weeks after diagnosis had a higher risk of death (HR, 1.9; 95% confidence interval [CI], 1.7–2.1; and HR, 1.1; 95% CI, 1.1–1.2, respectively). When adjusted for age, stage, race, year of diagnosis, and additional clinical and health system characteristics, surgery in the first and second weeks after diagnosis remained independently associated with death (HR, 1.4; 95% CI, 1.3–1.5; and HR, 1.1; 95% CI, 1.0–1.2, respectively). Mortality risk was significantly higher than baseline when surgery was performed in the eighth week postdiagnosis and worsened as time to surgery increased ( Figure 2 , A and B).
| Low risk | High risk | ||||
|---|---|---|---|---|---|
| Time to surgery, wk a | Patients | 5-y Survival (95% CI) | Time to surgery, wk a | Patients | 5-y Survival (95% CI) |
| 1 | 2057 | 73.0% (70.6–75.3%) | 1 | 1551 | 46.5% (43.5–49.5%) |
| 2 | 5665 | 85.0% (83.8–86.2%) | 2 | 3398 | 60.7% (58.8–62.6%) |
| 3 | 10,409 | 87.4% (86.5–88.2%) | 3 | 5153 | 66.9% (65.3–68.4%) |
| 4 | 12,593 | 86.2% (85.4–86.9%) | 4 | 5786 | 67.6% (66.0–69.1%) |
| 5 | 11,736 | 86.5% (85.7–87.3%) | 5 | 4994 | 67.1% (65.4–68.7%) |
| 6 | 9350 | 85.4% (84.4–86.4%) | 6 | 3975 | 66.7% (64.8–68.6%) |
| 7 | 6559 | 85.8% (84.7–86.9%) | 7 | 2675 | 65.0% (62.5–67.3%) |
| 8 | 4655 | 84.5% (83.1–85.9%) | 8 | 1905 | 66.3% (63.5–68.9%) |
| 9 | 3045 | 83.0% (81.0–84.8%) | 9 | 1224 | 62.5% (58.8–66.0%) |
| 10 | 2061 | 84.7% (82.4–86.8%) | 10 | 817 | 64.5% (60.0–68.7%) |
| 11 | 1367 | 82.1% (79.2–84.7%) | 11 | 638 | 66.0% (61.1–70.5%) |
| 12 | 972 | 79.7% (75.9–82.9%) | 12 | 398 | 60.5% (53.8–66.5%) |
| 13 | 673 | 81.9% (77.5–85.5%) | 13 | 296 | 55.9% (47.9–63.2%) |
| 14 | 540 | 81.0% (76.0–85.1%) | 14 | 243 | 57.8% (47.8–66.5%) |
| 15 | 402 | 78.6% (72.5–83.5%) | 15 | 197 | 57.7% (47.4–66.7%) |
| 16 | 326 | 71.1% (63.4–77.4%) | 16 | 153 | 49.6% (37.8–60.3%) |
| 17 | 250 | 77.2% (69.9–83.0%) | 17 | 119 | 57.5% (44.0–68.8%) |
| 18 | 201 | 72.9% (63.5–80.2%) | 18 | 113 | 51.0% (35.7–64.3%) |
| 19 | 174 | 69.7% (58.2–78.6%) | 19 | 71 | 47.6% (30.4–63.0%) |
| 20 | 141 | 77.0% (63.5–86.0%) | 20 | 72 | 61.2% (44.7–74.1%) |
| >20 | 898 | 74.5% (70.3–78.3%) | >20 | 462 | 55.7% (49.3–61.7%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


