INTRODUCTION
The cornerstone of gynecologic oncology fellowship training is the development of proficiency in radical pelvic surgery to adequately excise and stage disease and to address treatment or disease-related complications. From a surgical standpoint,
gynecologic oncologists were primarily recognized for expertise in radical and ultraradical procedures; however, surgical innovation has evolved with the goal of maintaining or improving survival while minimizing the extent of tissue removal and improving quality of life. As a result, gynecologic oncologists have gained expertise not only in radical pelvic surgery but also in gastrointestinal, genitourinary, and pelvic/vaginal reconstructive surgery.
Surgical procedures for preinvasive and invasive gynecologic neoplasia have become less radical over time where appropriate. Unfortunately, a small portion of patients will require an extensive and/or radical surgical procedure in order to cure primary disease. Occasionally, an ultraradical procedure such as the pelvic exenteration is required to address recurrent gynecologic malignancies. Therefore, reconstructive surgical procedures are often necessary with the outcome goals that include wound healing, decrease in acute and chronic morbidity, and restoration of anatomic form and function. Incorporating a pelvic/vaginal reconstructive procedure into radical pelvic surgery may be relatively straight forward but can often be quite complex considering that reconstruction may be enmeshed within a surgical case that includes multiple major surgical procedures that make up the entirety of the case. The gynecologic oncologist is best suited to orchestrate this multifaceted surgical process, and it is extremely important that the surgeon be well versed in the reconstruction options available in order that it is optimally planned. The objective of this chapter is to outline the most common reconstructive options and techniques available after radical and ultraradical pelvic surgery for gynecologic malignancies.
VAGINAL AND VULVAR RECONSTRUCTION
Radical surgery of the vagina and vulva often leaves a large defect where primary closure is not feasible. There is a wide range in the complexity of surgical situations that require reconstruction. Reconstructive goals include bringing in healthy tissue to fill the defect in order to enhance wound healing, and restoration of anatomic form and function. In these situations, reconstructive surgery is optimal when applied concurrently with the primary extirpative procedure but sometimes performed secondarily for repair of vaginal stenosis or vulvovaginal wound breakdown resulting from previous treatment complications. There are general guidelines that prioritize reconstructive options based on the size and location of the vaginal defect; however, the optimal reconstructive procedure will be dependent not only on the site and extent of the defect but also upon previous therapy applied to the pelvis and other intraoperative variables. Many reconstructive techniques are available, and most often, there is no one best option for any situation requiring reconstruction (
Table 55.1). Important considerations include not only the goals at the site of reconstruction but also the patient’s preoperative history and intraoperative condition, total length of the procedure, and potential donor site sequelae.
Patients with large pelvic soft tissue defects in addition to a vaginal defect are usually best managed with a large myocutaneous flap to provide biologic tissue bulk to the vacant pelvis, neovascularization, and vaginal reconstruction. Flaps should be outlined prior to the abdominal incision and potential ostomy sites taken into consideration. If possible, all pelvic reconstructive surgeries performed during an exenteration should be supplemented with an omental J-flap to form a pelvic lid in order to provide for additional neovascularization and to protect the small bowel. Considering the importance of minimizing severe postoperative morbidity, pelvic/vaginal reconstruction should be prioritized over complicated, timeconsuming, and higher-risk low rectal anastomosis and continent urinary conduits in the patients undergoing extensive total pelvic exenterations.
Patients with the largest defects are usually those undergoing infralevator total exenterations. A single large oblique rectus abdominis myocutaneous (RAM) flap is often sufficient to provide needed bulk in the pelvis, enhance closure of the perineal defect, and provide tissue for vaginal reconstruction. Supplemental flaps can be added to a single RAM that is adequate for providing bulk but not sufficient to complete the neovagina. Adding a full-thickness skin graft (FTSG) on a bed of omentum, supplementing with a pudendal thigh flap, and/or using a bulbocavernosus myocutaneous paddle to augment remaining longitudinal anterior or posterior defects are all very good supplemental options. Other alternatives to the RAM usually require bilateral procedures including the gracilis myocutaneous flap (GMF) or gluteal thigh flaps although they are not practical for addressing defects remaining after supralevator exenterations. Bilateral thigh flaps are better suited for extensive posterior defects. Potential advantages of these alternatives to the RAM include the opportunity for two surgical teams working simultaneously and avoiding any conflict with choosing the appropriate site for bilateral stomas, although many surgeons do not consider bilateral stomas to be a contraindication to the RAM.
Managing vulvar defects in a patient with no previous surgery or radiation therapy is relatively uncomplicated. Reconstruction for deep vulvar defects and/or radical partial excision of the vagina where it is important to bring in wellvascularized tissue to fill in dead space, provide neovascularization, and restore anatomic appearance and function can be more challenging especially if there is a history of previous surgery or irradiation. Ultraradical procedures that leave the patient with various combinations of pelvic, vaginal, and/or perineal defects provide the greatest challenge. Preoperative
planning is important, and the surgeon needs to be prepared to choose from a portfolio of reconstructive options based on the extent of the radical surgery and other intraoperative factors. Therefore, it is necessary that the gynecologic oncologist be proficient with multiple reconstructive options in order to optimally address the various vulvar/vaginal defects one may encounter.
SKIN GRAFTING
A graft is defined as any free tissue that is placed into a wound bed. Grafts differ from flaps in that they do not carry an intrinsic vascular supply. A skin graft must rely on the vascularity of the wound bed to optimize “graft take,” which is simply defined as adherence of the skin graft to the underlying wound bed with eventual neovascularization into the graft. Factors that impact skin graft survival include the thickness of the graft, whether it is a full-thickness or split-thickness skin graft (STSG); bacterial contamination of the wound bed; and postoperative complications such as hematoma formation or shearing of the graft. FTSG and STSG are both excellent options for vaginal reconstruction. FTSGs include the entire dermal layer. The advantage to an FTSG is an improved cosmetic appearance with a reduced incidence of secondary graft contracture. Disadvantages to FTSG include a lower possibility of “graft take” in wounds where bacterial contamination may be a concern. Both STSG and FTSG must be harvested from a donor site. Secondary donor site deformity usually constrains the size of the FTSG. STSG, on the other hand, can be harvested from a large square surface area and have varying degrees of thickness of dermis. Thin STSG have the highest likelihood of “graft take”; however, they are also most prone to secondary contraction as the wound heals. Balancing donor site issues, cosmetic concerns, wound contamination, and secondary graft contracture will lead to the optimal choice for graft placement.
Split-Thickness Skin Graft Harvest
Although STSG can be harvested from a variety of locations, the authors prefer to conceal the donor site. As such, the most common sites for harvest are the upper thighs. A small graft can be harvested using a hand-driven Weck blade, which is calibrated to harvest grafts that are 0.008, 0.010, and 0.012 inch thick. More commonly, a power-driven dermatome is used (e.g., Brown and Zimmer). A guard is selected according to the size needed. The thickness of the graft to be taken is then set. Despite this setting, the distance between the blade and instrument must always be checked by putting a Bard-Parker No. 15 blade between the blade and the guard, which approximates 0.015 inch.
Before harvesting the graft, the skin should be cleaned and mineral oil applied to lubricate the skin (see Steps in the Procedure box). The dermatome is turned on and engages the skin at an angle, exerting pressure on the skin. The dermatome is slowly pushed while the assistant helps by offering countertraction. Once the graft is harvested, it is placed in a saline-soaked sponge. If one wants to limit the size of the graft, it can be meshed. Grafts can be meshed in a variety of ways. An STSG can be “pie crusted” where a No. 15 blade is used to create full-thickness 1-to 2-mm holes within the main body of the graft. This allows for serous fluid to be evacuated from underneath the graft, which enhances adherence of the graft to the wound bed. STSGs can also be meshed with skin graft mesher (Zimmer), which produces a multiplied ratio of available surface area. Grafts are typically meshed 1:1, 1:2, or 1:3. Meshing to the latter ratio must be done with caution as the greater the number of mesh interstices, the more fragile the graft.
Grafts can be affixed with staples or with absorbable chromic gut or synthetic absorbable suture (3-0 or 4-0). The authors prefer suture placement, as staple removal in the clinic can prove quite uncomfortable for the patient. At present, there is no ideal way to treat the donor site. For a small area, Opsite is useful because it decreases pain; however, the major disadvantage is that the donor site weeps large amount of fluid in the ensuing 24 hours. An alternative is Xeroform gauze (3% bismuth tribromophenate) over the donor site, which is then allowed to dry over the wound. The authors’ preferred donor site dressing is Glucan II (Molnlycke Health Care, USA), a transparent dressing that can be suture affixed over the wound. This dressing does not dry out, as does Xeroform gauze, and also provides optimal relief from the discomfort of the open donor wound.
Full-Thickness Skin Graft Harvest
Theoretically, full-thickness skin grafts can be harvested from anywhere on the body where there is sufficient skin laxity to allow for excision and primary closure of the donor defect. One of the more common regions for FTSG harvest is the groin region above the level of the inguinal ligament. This donor site provides for a 5 × 10 cm graft harvest with primary closure. Grafts can also be harvested bilaterally. If a larger graft is necessary, the hypogastrum provides a full-thickness skin that can measure 10 × 20 cm depending on the degree of abdominal subcutaneous laxity.
FTSGs are harvested freehand using a Bard-Parker No. 10 or 15 blade (see Steps in the Procedure box). The blade incises the skin and is turned in an angle and elevated, leaving a small amount of dermis at the base. Any excess fat is then excised with Iris scissors. Unlike the STSG donor site, FTSG donor sites can be primarily closed at the time of graft harvest. Both skin edges are then elevated to obtain closure. Depending on tension at the closure, 3-0 or 4-0 monofilament, absorbable suture is used.
As with STSG, it is important to “pie-crust” an FTSG to allow for the exudation of serous fluid. Should fluid collect beneath the graft and wound surface, the graft will not be able to adhere to the bed. Areas of nonadherence will ultimately undergo necrosis. Meshing is usually not necessary for an FTSG. Once the FTSG has been affixed to the wound bed, a bolster must be placed. Bolsters can be fashioned in a variety of ways. The authors prefer cotton balls, mineral oil, Xeroform gauze, and a tie-over dressing with 2-0 silk sutures. Bolster dressings are typically left in place for 5 to 7 days and then removed in clinic.
Alternatives to Graft Placement
In some patients, use of an autologous graft placement may need to be delayed prior to achieving wound closure. Typically, such patients have a contaminated or clean-contaminated wound. Under such circumstances, the success of an STSG or FTSG viability may be unpredictable due to the degree of bacterial contamination. The use of xenograft (porcine) or cadaveric graft should be considered in these circumstances. Substitutes such as these will adhere to the underlying wound bed if the conditions are favorable. This strategy can be used as a test to evaluate the wound for receipt of either an FTSG or an STSG. Even in circumstances where a xenograft or cadaveric graft “takes” to the underlying wound bed, they will typically undergo resorption over a 3-week period allowing an opportunity for definitive closure.
INTEGRA Meshed Bilayer Wound Matrix (Integra Life Sciences Corp., Plainsboro, NJ) is an additional skin substitute that allows for assessment of the quality of the wound bed. INTEGRA consists of a porous matrix of cross-linked bovine tendon collagen and glycosaminoglycan and a semipermeable polysiloxane (silicone) layer. As such, the meshed bilayer allows drainage of wound exudate and provides a flexible adherent covering for the wound surface. The collagen-glycosaminoglycan biodegradable matrix provides a scaffold for cellular invasion and capillary growth. Over a 3-week period, the scaffold undergoes neovascularization. Once adherent to the wound bed, it provides an excellent recipient site for definitive autologous graft placement. INTEGRA not only allows for confirmation that a wound bed has been optimized for graft placement but also provides an additional several millimeters of soft tissue coverage if necessary.
Omental J-Flap with Skin Graft
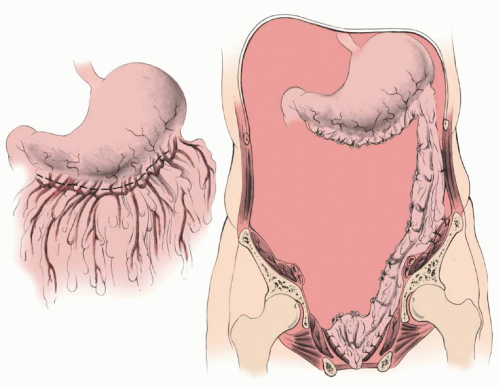
By modifying the omental flap that is normally used to close off the pelvic inlet after exenteration (
Fig. 55.1) (see Steps in the Procedure box), with or without low coloproctostomy,
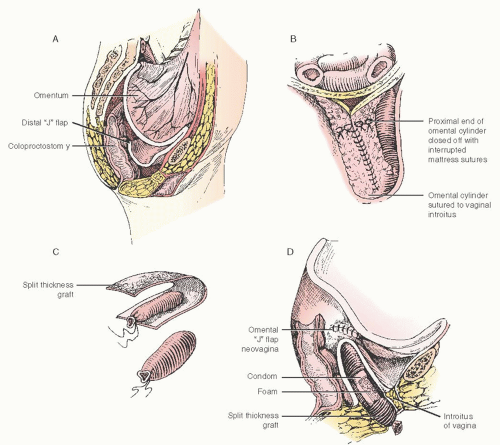
the surgeon can create an omental cylinder, providing anterior, posterior, and lateral walls for the neovagina (
Fig. 55.2A). When the cylinder is sutured to the introitus and lined with a skin graft (split or preferably full thickness) and expanded in the postoperative period by a soft rubber vaginal form, a satisfactory functional neovagina can be created (
Fig. 55.2B-D). The FTSG works well for neovaginal reconstruction with similar graft survival as compared to STSG, but is less likely to contract postoperatively compared with the STSG. However, when options are limited due to prior intervention or when faced with a bulky pedicle, for example, in obese patients, fullthickness grafts may be at higher risk of necrosis, favoring the STSG approach.
If construction of the neovagina is performed immediately following total pelvic exenteration, it is important to ensure that hemostasis in the pelvis is complete before applying the skin graft. If hemostasis is uncertain, the omental pocket for the neovagina should be packed with gauze. When hemostasis is secure and serous drainage has stopped, the packing can be removed and the graft can be taken as a secondary procedure and applied to a vaginal form and placed in the omental pocket.
In
Figure 55.2D, a sagittal section shows a patient who has undergone a total pelvic exenteration. The rectal stump was retained, and the descending colon was mobilized and brought down into the pelvis for a coloproctostomy. The omentum has been detached from the greater curvature of the stomach and brought down as a flap based on the left gastroepiploic artery. It is first sutured to the sacral promontory posteriorly, the pubic symphysis anteriorly, and pelvic walls laterally to form a pelvic lid. In patients who have insufficient omentum to form both the pelvic flap lid and walls of the neovagina, the omentum making the pelvic lid can be supplemented by the use of a sheet of synthetic, absorbable mesh with or without a skin graft lining as described by Elaffandi et al. Once the skin is placed in the neovagina, the graft eventually becomes almost indistinguishable from normal vaginal mucosa on histologic examination. After the development of estrogen receptors, the maturation index from cells of the graft can be influenced by the administration of systemic estrogen similar to normal vaginal mucosa.
In
Figure 55.3A, an FTSG has been harvested and rolled into a tube. It has been our practice to suture the graft over a soft foam mold (we use Model Magic (Crayola, Inc., Easton, PA)) that can be constructed to fit the individual defect giving the surgeon flexibility in choosing the length and diameter of the stent. The skin graft is folded over the vaginal form, and the edges of the graft are sutured with a running fine synthetic absorbable suture (
Fig. 55.3B). The graft and stent are inserted into the omental tube so that the epithelial surfaces are opposed. The labia majora are sutured together for 7 days to allow for the skin graft to “take” and to prevent the stent from being extruded out of the neovagina. For a period of 6 months thereafter, a vaginal stent should be worn at all times, except during intercourse and douching, in order to prevent graft shrinkage, which leads to vaginal stenosis. After 6 months, the vaginal form is worn only at night if the patient is not sexually active. The omental J-flap with a skin graft lining has proven to be a reliable technique for vaginal reconstruction and is associated with minimal added morbidity and can result in satisfactory vaginal function.
Skin Grafting of Vulvar Defects
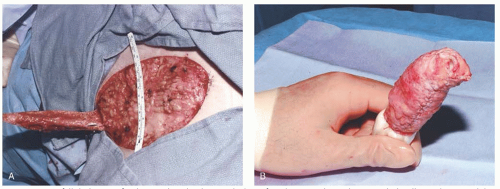
Most vulvar defects that are the result of a simple excision (wide local excision, partial or total vulvectomy) can be closed primarily, especially if the surgical field has not been treated with radiation and the lesion is less than 4 cm. Radical procedures for vulvar malignancies result in very deep defects where a skin graft will not be sufficient to close the defect if reconstruction is required. Skin grafting is useful when it is necessary to perform a superficial but large surface “skinning” vulvectomy that often involves resection of perineal and perianal skin in addition to the vulva. These are large surface area resections usually performed for extensive VIN or Paget disease and can be difficult or impossible to close primarily leading to wound dehiscence and/or disfigurement. The procedure and reconstruction has been described by Caglar and colleagues and also Dettenmaier et al. Reconstruction by placing a skin graft over the subcutaneous tissue is preferable to allowing such a large defect to heal by secondary intension.
Preparation of the donor site, harvesting the STSG, and care for the donor site are described above. The recipient site
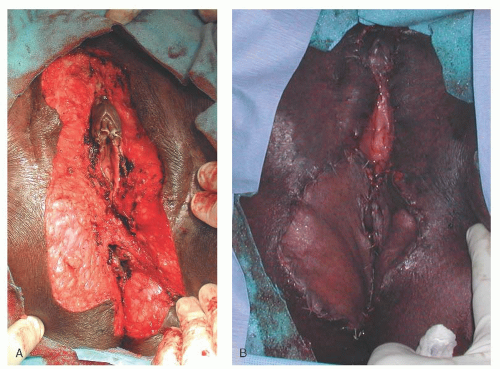
is prepared by ensuring meticulous hemostasis (
Fig. 55.4A). Meshing is recommended considering the risk for infection and serous drainage collections in these larger external and perianal wounds. Larger slits are designed to accommodate the vaginal introitus and anus. The edges of the meshed graft are sutured to the edges of the vulvar defect with fine absorbable suture in an interrupted fashion (
Fig. 55.4B). Moist gauze is then packed and secured over the defect as a bolster to ensure that the graft is in contact with the subcutaneous tissue. It is important that the patient is immobilized for 5 to 7 days to prevent the graft from shearing from the recipient site. Therefore, deep vein thrombosis prophylaxis should be instituted, and the patient may require a Foley catheter and/or a rectal tube while at bed rest. The secured packing is carefully removed so as to not inadvertently disrupt the adherent graft. Dainty and others have reported the use of fibrin tissue adhesives, and wound vacuum-assisted closure devices has been reported to improve graft viability during vulvovaginal reconstruction.
Tissue Transposition Flaps
Adjacent tissue transfer flaps or skin flaps are sufficient for the majority of situations encountered that require reconstruction after radical vulvar surgery except for the most extensive resections. Tissue transposition flaps can also be used as alternatives to skin grafts and myocutaneous flaps for vaginal reconstruction. These local flaps can be supplied by random vascularization from the underlying subcutaneous vascular network or derive their blood supply from a single-vessel or defined vascular territory. Tissue transposition flaps are relatively easy to perform, and provide well-vascularized tissue with needed bulk for deeper vulvar defects that are not amenable for primary closure or superficial skin grafting. A unilateral or bilateral rhomboid flap as described by Burke et al. is usually sufficient for most vulvar defects. Other options that may be used as an alternative to a myocutaneous flap, especially for extensive vulvar defects in a radiated surgical field, include the medial thigh and gluteal V-Y advancement flap and rotational flap. Monstrey and colleagues have described the use of a very versatile pudendal thigh flap in 19 patients. Bulbocavernosus flaps have also been used for many years to repair various vulvovaginal defects, but Green and the group from the Cleveland Clinic have recently revisited this technique for construction of a neovagina following total pelvic exenteration
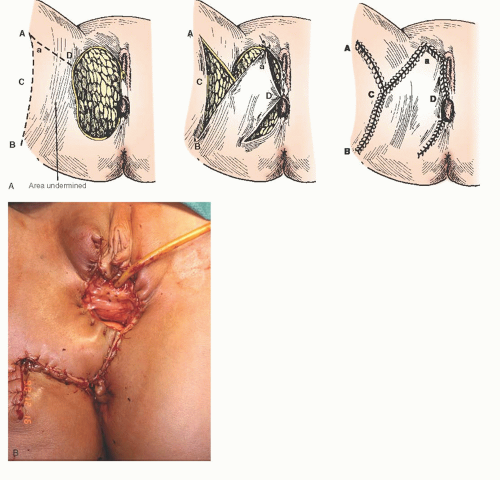
Rhomboid Flap
Large lateral vulvar defects can be satisfactorily closed with a unilateral rhomboid flap, while perineal defects of most any size are usually best closed with bilateral flaps. The rhomboid flap is raised according to the measured vulvar or perineal defect and best designed with a 1:1 height to width ratio. Conditions that may compromise the vascular supply such as previous irradiation and/or surgery in the area of the skin flap may also compromise graft viability. In addition to adequate blood supply, successful healing of these flaps is dependent on positioning and closure without tension.
A unilateral flap is illustrated in
Figure 55.5A, B. Prior to making the incisions to create the flap, the defect is measured and then a design is marked to the appropriate size so that it will be able to be rotated into and fill the defect without tension
once it is sutured into place. The incision is then made through the skin and subcutaneous tissue down to the level of the fascia. Next, the deep aspect of the subcutaneous tissue is dissected off of the fascia, starting from the apex and back toward the base of the flap. In order to take tension off of the flap, the subcutaneous edges of the flap are approximated to the subcutaneous edges and base of the defect with interrupted 3-0 polyglycolic absorbable (PGA) suture. Undermining of the deep subcutaneous tissue adjacent to the surgical defect may assist wound closure without tension (see Steps in the Procedure box). A subcutaneous drain with suction may be placed for large defects for drainage and elimination of dead space. The skin is closed with interrupted 3-0 PGA in a mattress fashion.
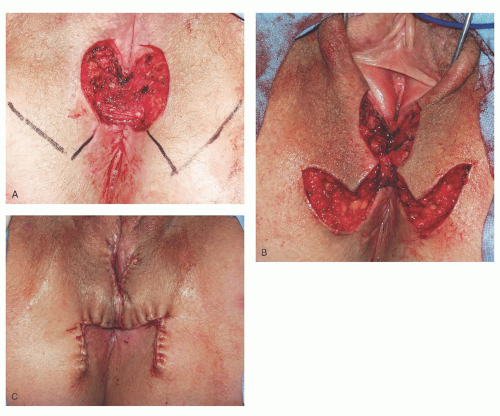
Most anterior defects can be closed primarily; however, radical vulvar surgery often leaves a significant posterior perineal defect. While primary closure is often possible, close approximation of the vagina and anus is not desirable. Due to their bulk, large rotational skin flaps and myocutaneous flaps are not practical in this situation. Bilateral rhomboid flaps are usually the ideal reconstructive option to fill these defects. The flaps are designed according to the size of the defect with the goal of meeting in the midline without tension (
Fig. 55.6A). The mobilized flaps are stabilized by suturing the subcutaneous fat to the base of the defect and to the subcutaneous fat at the defect edges and to the “sister” flap in the midline (
Fig. 55.6B). The skin is closed with interrupted 3-0 PGA in a mattress fashion (
Fig. 55.6C). Usually, a drain is not needed in this situation.
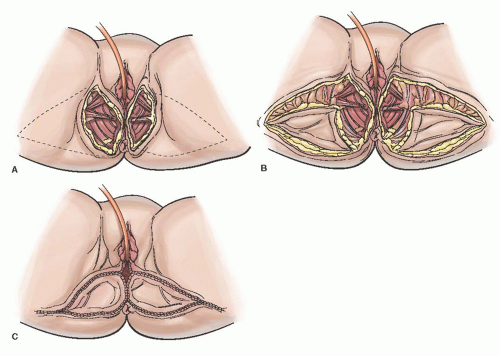
V-Y Advancement Flap
The V-Y flap is an excellent option for large unilateral and bilateral vulvar defects. These flaps are technically easier to perform compared to the GMF, have a reliable vascular supply, are sensate, and can still fill a relatively large defect. The flap is dependent on random perfusion for its vascular supply from the pelvis and medial leg muscles. The flap is designed in the shape of a triangle and usually harvested from the medial thigh or the gluteal fold. The flap is designed with the apex either at the gluteal fold or on the medial thigh with the base at the wound defect (
Fig. 55.7A). The skin and subcutaneous tissue is incised down to the underlying fascia, and the flap is mobilized according to the size of the defect, which results in the shape of a “V” (
Fig. 55.7B). The flap is advanced or “slid” over to the medial edge of the defect without tension and sutured in a similar fashion as described for the rhomboid flap. The resulting suture line now has the appearance of a “Y” (
Fig. 55.7C). Immobilization and drains can be considered depending on the size of the flap and extent of subcutaneous dissection.
Pudendal Thigh Flap
The pudendal thigh fasciocutaneous flap is based on blood supply from the internal pudendal artery and can be designed as a rotational flap with a peninsular base or as an island flap with a pedicle when there is a need for tunneling or increased flap mobility. When the integrity of the blood supply is in question, the nonisland version of this flap should be used to take advantage of random subcutaneous blood supply. The flap is reliable, is easy to harvest, leaves an inconspicuous donor site scar, and is less bulky than is a myocutaneous flap. Unilateral flaps are quite useful for filling partial vaginal defects that are posterior or augmenting unilateral myocutaneous flaps during total vaginal reconstruction. Bilateral flaps may be sufficient for total vaginal reconstruction. The flap is sensate and does have the potential for hair growth.
The flap is outlined over the labial-crural fold and is usually 5 to 6 cm in width and up to 15 cm in length (
Fig. 55.8A), but is usually designed on a 2:1 length-to-width ratio. The skin and subcutaneous tissue are incised down to and through the deep fascia (
Fig. 55.8B). A posterior-based rotational flap is then mobilized and can be rotated into the vaginal defect and sutured in multiple layers to eliminate dead space and tension (
Fig. 55.8C, D). A subcutaneous drain is recommended for the large donor site prior to closing.