Fig. 3.1
Burch colposuspension: The initial suture is at the urethrovesical junction and the second suture is approximately 1 cm caudal. Care is taken to place these sutures at least 4 mm lateral to the urethra. The sutures are then placed through Cooper’s ligament
Laparoscopic/Robotic
The patient is placed in a dorsal lithomtomy position with legs in stirrups. A 16 Fr. Foley catheter is placed at the beginning of the procedure. Monitors, typically two for the surgeon and assistant surgeon, are placed at the patient’s feet. The two midline trocars are for both introduction and extraction of curved needles and passage of the laparoscope. The 5 mm trocars are placed laterally at the border of the rectus muscles, at the level of the suprapubic 12 mm trocar (the distance between the pubic symphysis and 5 mm trocars would be no less than 4 fingerbreadths). This distance allows for adequate access to the space of Retzius.
Once any concomitant procedures are completed (e.g., hysterectomy, prolapse repair) the space of Retzius is entered. Blunt and sharp dissection is used to expose the pubic symphysis and Cooper’s ligament. Also dissected out and exposed are the lateral pelvic sidewall, obturator neurovascular canal, ischial spine and arcus tendinius, arcus of the levator ani, and the paravaginal fascia. If indicated, a paravaginal repair can be performed if there is a lateral cystocele defect.
At this point, Ethibond No. 0 sutures are placed in the same fashion as described for the open approach. After the sutures are tied down, cystoscopy is performed after indigo carmine is injected intravenously to check for ureteral efflux, ruling out any obstruction. The 12 mm trocras are closed in standard fashion with 2-0 absorbable suture. The 5 mm trocar facsia does not need to be closed. The patient may undergo a voiding trial in the recovery room according to individual surgeon preference.
Outcomes
The Burch colposuspension has been shown to outperform pharmacotherapy, conservative management, needle suspensions, Marhsall–Marchetti–Krantz procedure, and anterior colporraphy [9]. A recent Cochrane review of open Burch colposuspensions reported an overall success rate of 69–88 %. This same meta-analysis had separately reviewed 12 trials comparing open approach versus laparoscopic approach and found no statistically significant difference in patient-reported incontinence and the 1-year and 5-year follow-up periods.
There have been studies that have evaluated the long-term success rates of the open Burch colposuspension. Sivaslioglu et al. reported an 84 % success rate at 7 years in their series of 262 patients [10]. The Burch colposuspension is a safe and effective surgical option for SUI, largely considered for women undergoing a concomitant open or laparoscopic procedure such as pelvic organ prolapse repair [11].
Complications
As any open or laparoscopic abdominal procedure, there are common risks including bleeding, infection, erosion of materials involving the bladder, injury to abdominal organs, and hernias [12]. The main long-term issues at hand center around voiding dysfunction and pelvic organ prolapse postoperatively. These pelvic floor issues include detrusor overactivity, urinary retention, and formation of enterocele/rectocle.
Detrusor Overactivity
Many studies have reported differing rates of de novo detrusor overactivity. The mechanism of the dysfunction is widely thought to be secondary to increased elevation of the vagina, and ostensibly the bladder trigone at urethropexy. This therapy further emphasizes the importance of stabilization versus elevation as an important factor in the success of the Burch colposuspension. One of the earlier reports that came from Stanton et al., whose group reported postoperative urodynamic-proven de novo detrusor overactivity, demonstrated a rate of de novo detrusor overactivity at 18.5 % [13]. In Langer et al.’s 10-year follow-up study, the incidence of de novo detrusor overactivity was 16.6 %. Voiding dysfunction appeared within the first year in 70.5 % of the patients ultimately diagnosed with de novo detrusor overactivity [14].
Urinary Retention
In reviewing the literature the on incidence of long-term urinary retention, the authors acknowledge that there is not a great deal reported. Alcalay et al. reported four of the 366 patients who underwent the Burch colposuspension required urethrolysis postoperatively [15]. Although Feyeriesl reported a 16 % rate of residual >60 ml in their patient population at 5- to10-year follow-up, the authors do not report on any patients with residuals greater than 150 ml. Suffice it to say, there is a risk of urinary retention in the Burch colposuspension technique, albeit most likely a low risk.
Enterocele/Rectocele Formation
As discussed in the technique section, the goal of Burch colposuspension is stabilization, not elevation. In early series, the risk of enterocele or rectocele formation is widely thought to be secondary to over-elevation of the vaginal wall [16]. Keeping this in mind, more recent series have lower rates of this anatomic sequelae by avoiding excessive elevation.
Periurethral Bulking Agent
The first description of the injection of a periurethral agent for the management of stress urinary incontinence came from Murless in 1938. The substance used was sodium morrhuate. Following that, may others published experiences with a wide variety of injectables, including paraffin wax, sclerosing agents, polytetrafluoroethylene, collagen, autologous fat, silicone, and stem cells. Despite the significant presence of injectable agents in urologic practice, there have been very few well-designed published studies evaluating the efficacy of this therapy.
The patient selection for this procedure consists of patients with ISD and normal detrusor function. The urodynamic cutoff for Leak Point Pressure (LPP) is typically 60 cm H2O [17]. The success in ISD patients is thought to be secondary to the mechanism of action which is thought to be a result of increased area and pressure transmission ratio. This would ostensibly prevent the bladder neck or proximal urethra from opening under stress. Patients may also have hypermobility of the urethra, and still have their ISD component addressed with an injectable agent [18]. In this section, we will review the technique and outcomes of this therapeutic modality. In addition to these indications, urethral bulking agents are also indicated in patients who are young and desire more children, poor surgical candidates, persistent SUI after anti-incontinence procedure, and SUI with poor bladder emptying.
Surgical Technique
The most common environment for this procedure is under local anesthesia in an outpatient basis. There are two main approaches—transurethral and periurethral. The agent is typically placed submucousally or into the lamina propria. The injectable can be placed at the bladder neck or the proximal urethra. The typical sites of implant are the 3 and 9 o’clock positions. The size of the needle is dependent on the injectable agent. The propose mechanism of action is to achieve coaptation of the urethra during the storage phase, with maintenance of this coaptation when there is an increase in abdominal pressure transmitted to the bladder with a valsalva maneuver.
Periurethral
The patient is placed in dorsal lithotomy position. Local anesthesia is injected in the 3 and 9 o’clock positions 3 mm lateral to the urethral meatus. A 30° cystoscope is introduced after local anesthesia is injected. The periurethral needle is then placed lateral to the urethral meatus (same site as local injection) and advanced to the bladder neck/proximal urethra. The agent is injected in the 3 o’clock position on the right, followed by 9 o’clock position on the left. The goal is to create blebs that meet in the midline, akin to prostatic lateral lobes (Fig. 3.2). If there is any mucosal leakage of the injectable agent from a rent in the mucosa, which can be seen with a transurethral technique, the needle can be repositioned and agent reinjected. At this point, the patient is asked to valsalva to evaluate for SUI. If there is still SUI, more of the injectable agent may be injected. Once completed, the patient is asked to void and residual is checked. If in urinary retention, a small caliber catheter, 8 or 10 Fr., is inserted. A theoretical benefit of the periurethral technique is the avoidance of mucosal leakage and local bleeding that may occur with transurethral needle injection.
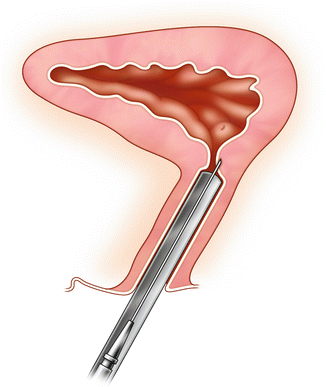

Fig. 3.2
Bulking agent injection for SUI: The agent is injected in the 3 o’clock position on the right, followed by 9 o’clock position on the left. The goal is to create blebs that meet in the midline, akin to prostatic lateral lobes
Transurethral
The set up is quite similar to the periurethral approach. Local anesthesia may be instilled via the urethra. Needles specific to the injectable agent or generic needles may be used to inject transurethrally in the same locations described in the above section. A proposed advantage of this technique is better visualization of the injected material compared to the periurethral technique.
Outcomes
There have been many agents that have been used over the years as periurethral bulking materials. For the purposes of remaining current, the authors will review outcomes of bulking agents that are available at the time of publication of this text.
Macroplastique® (Uroplasty Inc, Minneapolis, MN) is a nonbiodegradable hydrogel composed of vulcanized polydimethylsiloxane elastomer suspended in a water-soluble carrier gel (polyvinylpyrrolidone). The agent does not require preadministration testing. The bulking agent can be administered with a 18 gauge endoscopic needle or a proprietary nonendoscopic transurethral injection device called the MIS (Macroplastique® Implantation System, Uroplasty Inc, Minneapolis, MN). The device is a mutichanneled needle positioning tool angled needle entry point with 6, 2, and 10 o’clock position. The typical volumes of injection are 2.5 ml, 1.5 ml, and 1.5 ml, respectively. There have been many studies reporting the success rates of Macroplastique. Most recently, there was a multicenter trial of 247 patients randomized to Macroplastique or Contigen® (collagen) (Bard Medical, Murray Hill, NJ). At 12 months follow-up, improved and dry/cure rates were 61.5 % and 36.9 % in patients injected with Macroplastique versus 48 % and 24.8 % in patients injected with Contigen.
Durasphere® (Boston Scientific, Natick, MA) is made of pyrolytic carbon-coated zirconium beads suspended in a water-based carrier gel composed of 2.8 % glucan. Due to concern for the potential of migration, Durasphere was designed with large-caliber particles (>80 m) in order to obviate this issue. There are 1 ml and 3 ml formulation. That having been said, there have been reports published on local periurethral and local lymphatic migration [19]. The first generation of Durasphere was plagued by issues of difficulty with injection using a proprietary 18 gauge needle with standard endoscopic instruments. Dusrasphere EXP was developed, which included a reformulated carbon bead size and carrier gel to be injected with a customized, side-firing 18-gauge or 20-gauge needle. One of the larger randomized trials of 355 women compared Durasphere to bovine collagen. The study showed no significant difference in outcomes: 80.3 % treated with Durasphere and 69 % treated with collagen were improved by one or more continence grade at 12 months [20].
Coaptite® (Boston Scientific, Natick, MA) is composed of particles of calcium hydroxylapatite ranging in diameter from 75 to 125 μ suspended in an aqueous gel carrier composed of sodium carboxymethylcellulose and glycerin. There is a 1 ml formulation. The injection can be performed with standard endoscopic instruments with a supplied 21-gauge rigid injection needle, available in end-firing and side-firing capability. One of the largest multicenter randomized trials compared Coaptite to cross-linked collagen in 296 women. Patients treated with Coaptite had 63.4 %, versus 57 % of those treated with collagen, rate of improvement of 1 Stamey incontinence grade or more; this was statistically significant. The study also demonstrated that fewer patients treated with Coaptite required repeat injections compared to collagen patients, 62 % versus 74 % [21].
Complications
Complications have been reported in all injection agents currently available in the US market. Macroplastique adverse events have included dysuria (short-lived, self-limited), frequency, and hematuria in many patients. Urinary retention has been reported in 6–10 % of patients injected with Macroplastique [22]. In addition to the common adverse events listed above for Macroplatique, Dursaphsere has been shown to result in noninfectious periuerthral abscess formation and urethral prolapsed [23]. There have also been case reports of urethral prolapsed after Coaptite injection [24].
Pubovaginal Sling
First introduced at the beginning of the twentieth century, the pubovaginal sling procedure has remained an excellent, viable option for the management of SUI. The materials used include both synthetic and biologic options. A common synthetic described in the literature is polypropylene. Biologic have included autografts (rectus fascia, fascia lata, and vaginal wall), allografts (fascia, dermis, and dura mater), and xeongrafts (procine or bovine). Although there are many published studies evaluating all of these options, autologous rectus fascia is the most commonly used approach and represents the greatest body of literature (this will be the focus of this section). Before the widespread application of the synthetic midurethral sling, pubovaginal slings were largely considered the gold standard of care for the management of SUI.
Surgical Technique
The patient is placed in a dorsal lithotomy position and the abdomen and vagina are prepped and draped in standard fashion. A transverse lower abdominal incision is made 2 cm above the pubic symphysis approximately 7 cm in length. Dissection is carried down to the rectus fascia, which is cleared of overlying fat. A 2 × 8 cm portion of the fascia is marked. The fascia is then harvested with either sharp or electrocautery dissection (Fig. 3.3a, b). Once the fascia is harvested, the defect is closed with 0 delayed absorbable suture. With a Foley catheter in place, the bladder neck is identified. A midline, vertical incision is made after the anterior vaginal wall is hydrodissected with a mixture of 1 % lidocaine with 1:200,000 epinephrine solution. A tunnel is then created to the retropubic space using sharp and blunt dissection. The dissection is carried to the level of the posterior rectus abdominis facsia. Pereyra needles are then passed suprapubically 2 cm on either side of the midline into the vaginal incision. A cystoscopy is performed with both 30 and 70° lenses to rule out injury to the urethra or bladder.
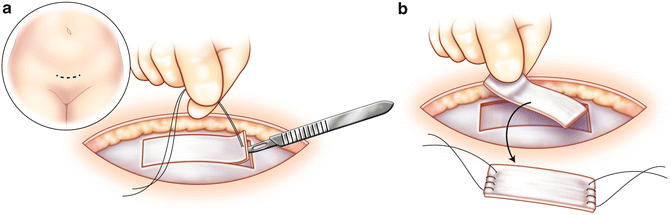

Fig. 3.3
Pubovaginal sling: A 2 × 8 cm portion of the fascia is marked (a). The fascia is then harvested with either sharp or electrocautery dissection (b)
The harvested fascia is then prepared for implantation. 0 prolene sutures are placed on either side. The sutures are then placed through the eyelet of the Pereyra needles and brought through the abdominal wall bilaterally (Fig. 3.4a, b, c). The sutures are then tied over the abdominal wall (on top of one finger to avoid overtensioning). The anterior abdominal subcutaneous layer is closed with 2-0 absorbable suture and skin with 4-0 absorbable suture. The vaginal wall is closed with 2-0 absorbable suture. A vaginal packing is placed along with a 16 Fr. Foley catheter. The patient will have a voiding trial in 5–7 days. If there are elevated residuals (>150 ml), the patient will perform intermittent straight catheterization until her residuals return to normal.
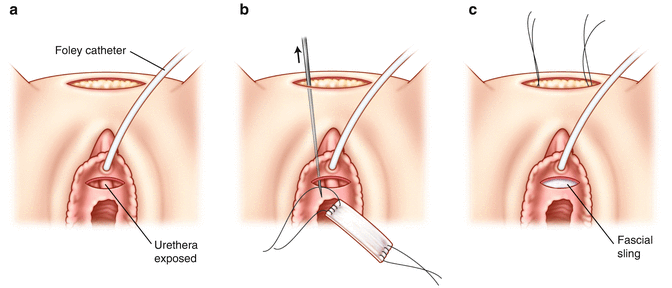

Fig. 3.4
Pubovaginal sling: the anterior vaginal wall dissection is performed (a). 0 prolene sutures are placed on either side (b). The sutures are then placed through the eylelet of the Pereyra needles and brought through the abdominal wall bilaterally (c)
Outcomes
The SISTeR trial was the largest randomized control trial reported in the literature evaluating the efficacy of autologous rectus fascia pubovaginal sling. The Urinary Incontinence Treatment Network (UITN) designed and executed this multicenter trial. The study, which consisted of 655 women, compared outcomes of patients randomized to autologous rectus fascial pubovaginal sling and Burch colposuspension. The success rates, defined as no self-reported symptoms of SUI, was higher in the pubovaginal sling group than the Burch colposuspension group, 66 % and 49 % respectively. This reached statistical significance with a P < 000.1. The same group went on to publish their 5-year follow-up data on 482 patients. The authors found that there were significant declines in continence in both groups.However, there were higher continence rates in the pubovaginal sling group compared to the Burch colposuspension group, 30.8 % and 24 % respectively (P = 0.002). Although patient satisfaction decreased for both groups, rates of patient satisfaction were still higher in the pubovaginal sling group compared to the Burch colposuspension group after 5 years, 83 % and 73 % respectively (P = 0.03) [25].
There are 14 other published RCTs looking at pubovaginal slings, a majority utilizing autologous rectus fascia. All consistently demonstrated the efficacy of the pubovaginal sling in the management of SUI. The pubovaginal sling has also seen another indication in light of recent complications noted with midurethral synthetic slings, namely lower urinary tract erosion [26]. In addition, pubovaginal slings have become the transvaginal anti-incontinence procedure of choice for concomitant repairs of urethral diverticula and urethrovaginal fistulas [27].
Complications
There are known complications related to pubovaginal slings. Some common adverse events include urinary tract infections (UTI) (48 %), voiding dysfunction (14 %), and postoperative urge incontinence requiring treatment (27 %) [25]. Of note, adverse events were more common in studies which used synthetic material for pubovaginal slings.
Midurethral Synthetic Slings
In 1995, Ulmsten first introduced the synthetic midurethral sling procedure [28]. In the last 3 decades, this procedure has become the most commonly employed for the treatment of SUI [29]. Proponents of this surgical option would argue the reason for this overwhelming popularity is due to short learning curve, brevity of the procedure, and low morbidity. In addition, there have been many studies that have demonstrated the excellent long-term durability and success rate of the procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


