Streptococcus Group A Infections
Mark R. Schleiss and Edward L. Kaplan
Streptococcus pyogenes infections were likely responsible for the apparent scarlet fever epidemic described by Hippocrates in the 5th century BC. The history of S pyogenes has been the subject of a comprehensive review.1 The first modern description of streptococcal infection was the demonstration of the organism in patients with erysipelas and wound infection in 1874. The organism was designated Streptococcus pyogenes by Rosenbach in the late 19th century. In the early 1930s, Rebecca Lancefield’s classification of the β-hemolytic strains into characteristic distinct serogroups led to the recognition that serogroup A isolates (S pyogenes) were the streptococcal strains most commonly responsible for pharyngitis and impetigo/pyoderma. Streptococcus pyogenes is one of the most important infectious agents encountered in clinical practice causing infections of the upper respiratory tract and of the skin, that causes a variety of severe systemic infections, including toxic shock syndrome and life-threatening skin and soft tissue infections. Infection with this pathogen is also causally linked to two serious nonsuppurative complications, acute rheumatic fever and acute glomerulonephritis.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Streptococcus pyogenes is highly communicable. Respiratory droplet spread is the major route for transmission of strains associated with upper respiratory tract infection, although skin-to-skin spread is known to occur with strains associated with pyoderma. Foodborne outbreaks are not rare and often are associated with egg-containing foods. Although uncommon, nursery outbreaks of group A streptococcal infections have been reported. The incidence of pharyngeal infection with group A streptococci is highest in children ages 5 to 15 years. Indeed, group A streptococcal pharyngitis has been described as an “occupational disease” of school-aged children. Streptococcus pyogenes also has the potential to produce outbreaks of disease in younger children in group daycare. In temperate zones, pharyngeal infection is most common during late autumn, winter, and early spring. Group A streptococcal skin infections occur most frequently during the summer in temperate climates but can occur year-round in warmer climates.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Streptococci are gram-positive cocci that tend to grow as pairs and chains. When cultured on sheep or horse blood agar plates, a characteristic zone of complete hemolysis (β-hemolysis) is observed. Streptococcus pyogenes (group A streptococci) may be identified either by serologic means or latex agglutination techniques. Additional typing of group A streptococci for epidemiologic purposes is based on variation in the M and T proteins and emm genes (see below). The somatic cellular constituents as well as the extracellular enzymes and toxins responsible of S pyogenes are responsible for many of pathogenic effects observed in vivo. These also are summarized in eTable 285.1  .2,3 A complete and current listing of recognized types of group A streptococci is available at the Centers for Disease Control (CDC) Web site (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm). The major virulence factor of the organism is the M protein. This protein is anchored to the cell membrane and transverses and penetrates the cell wall. Functionally, the M proteins inhibit phagocytosis, which is a primary virulence mechanism for survival in tissues. Immunity to M protein is a key determinant in protection against infection. In the nonimmune host, M protein mediates its antiphagocytic effect by inhibiting activation of the alternate complement pathway. Genes related to the M protein gene (emm) are called the M gene superfamily and include immunoglobulin-binding proteins. Other streptococcal cell wall antigens are important in the pathogenesis and the epidemiologic typing of S pyogenes. Most strains are enveloped in a hyaluronic acid capsule that serves as an accessory virulence factor by inhibiting phagocytosis. Lipotechoic acid and protein F are cell wall constituents that play roles in the adherence of S pyogenes to fibronectin on the surface of human epithelial cells, an important event in the initiation of the infectious process. Serum opacity factor (OF) is a lipoproteinase associated with M protein that is useful in classifying strains that are not identifiable by M typing.
.2,3 A complete and current listing of recognized types of group A streptococci is available at the Centers for Disease Control (CDC) Web site (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm). The major virulence factor of the organism is the M protein. This protein is anchored to the cell membrane and transverses and penetrates the cell wall. Functionally, the M proteins inhibit phagocytosis, which is a primary virulence mechanism for survival in tissues. Immunity to M protein is a key determinant in protection against infection. In the nonimmune host, M protein mediates its antiphagocytic effect by inhibiting activation of the alternate complement pathway. Genes related to the M protein gene (emm) are called the M gene superfamily and include immunoglobulin-binding proteins. Other streptococcal cell wall antigens are important in the pathogenesis and the epidemiologic typing of S pyogenes. Most strains are enveloped in a hyaluronic acid capsule that serves as an accessory virulence factor by inhibiting phagocytosis. Lipotechoic acid and protein F are cell wall constituents that play roles in the adherence of S pyogenes to fibronectin on the surface of human epithelial cells, an important event in the initiation of the infectious process. Serum opacity factor (OF) is a lipoproteinase associated with M protein that is useful in classifying strains that are not identifiable by M typing.
Group A streptococci also produce a large variety of extracellular enzymes and toxins. The family of streptococcal pyogenic exotoxins (SPE) includes SPEs A, B, C, and F. These toxins are responsible for the rash of scarlet fever. They also produce other pathogenic effects, including pyrogenicity, cytotoxicity, and enhancement of susceptibility to endotoxin. Streptococcal pyogenic exotoxin B is a precursor for a cysteine protease that functions as a virulence factor. Isolates associated with streptococcal toxic shock syndrome encode certain SPEs (A, C, and F) functioning as superantigens. These antigens induce a marked febrile response, induce proliferation of T-lymphocytes, and induce synthesis and release of multiple cytokines, including tumor necrosis factor, interleukin 1-beta, and interleukin-6. Streptococcus pyogenes also elaborates two extracellular hemolysins, streptolysin O and streptolysin S. Streptolysin O is toxic to eukaryotic cells, including myocytes. Streptolysin S is capable of damaging polymorphonuclear leukocytes and subcellular organelles. Other extracellular products elaborated by S pyogenes include deoxyribonucleases (DNAses A-D), hyaluronidase, and streptokinase.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Streptococcal Pharyngitis
Acute pharyngitis represents one of the most common reasons children are seen by primary care physicians. Yet, in spite of the common nature of the infection, the diagnostic and therapeutic approach to the child with pharyngitis or tonsillitis remains a source of controversy.5-7 Some of the points debated among clinicians include the following: (1) Which children should be tested for streptococcal pharyngitis? (2) How should children be tested for streptococcal pharyngitis? (3) What antibiotic therapeutic approach(s) should be used for streptococcal pharyngitis?
In general, decisions about laboratory testing and antibiotic therapy should be made only after careful consideration of clinical findings and epidemiologic considerations. The most important historical information in the evaluation of the complaint of sore throat is that of the presence or absence of other symptoms of upper respiratory infection. Children with bona fide streptococcal pharyngitis rarely if ever have cough, rhinorrhea, or symptoms of a viral upper respiratory infection (URI), and the diagnosis of streptococcal pharyngitis can almost always be excluded with the clinical findings of coryza, hoarseness, cough, or conjunctivitis. Although these are important exclusionary criteria, the pediatrician must be aware that the signs and symptoms of streptococcal pharyngitis may be nonspecific and vary greatly depending on the age of the patient, the severity of the infection, or the seasonal timing of the illness. Young infants do not present with classic signs and symptoms of pharyngitis. Streptococcal upper respiratory tract infections in infants and toddlers may instead be characterized by low-grade fever, anorexia, and a serous nasal discharge (so-called streptococcosis). Conversely, some patients may be toxic, with high fever, malaise, headache, and severe pain on swallowing. Vomiting and abdominal pain may be prominent early symptoms, simulating gastroenteritis or even acute appendicitis.
On physical examination, children with group A streptococcal pharyngitis classically demonstrate tonsillopharyngeal erythema, a red edematous uvula, palatal petechiae, and tender anterior cervical adenitis. Typically, tonsils are enlarged and erythematous with patchy exudate on the surface. The papillae of the tongue may be red and swollen (so-called strawberry tongue). Cutaneous petechiae have even been noted, and a scarlatiniform rash may be present (Fig. 285-1). When the characteristic rash of scarlet fever is present, a clinical diagnosis can be made with increased confidence. However, it is difficult to consistently make the diagnosis of streptococcal pharyngitis on clinical grounds alone. Therefore, even the most experienced clinician should consider bacteriologic confirmation of the diagnosis whenever possible. Obtaining appropriate diagnostic testing for group A streptococcal pharyngitis before therapy is instituted may prevent unnecessary antibiotic prescriptions for viral pharyngitis.8
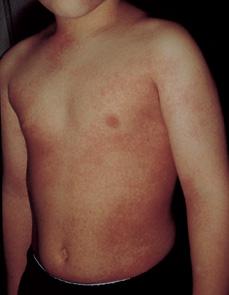
FIGURE 285-1. Erythematous scarlatiniform rash of scarlet fever. (From Knoop KJ, Stack LB, Storrow AB, Thurman RJ. The Atlas of Emergency Medicine. 3rd ed. New York: McGraw-Hill, 2010. Photo contributor: Lawrence B. Stack, MD.)
What kind of bacteriologic confirmation of the tentative diagnosis of streptococcal pharyngitis is appropriate? If performed correctly, a throat swab cultured on a blood agar plate has a sensitivity of 90% to 95% in detecting the presence of S pyogenes in the pharynx. The specimen should be obtained from the surface of both tonsils/tonsillar fossae and from the posterior pharyngeal wall. Concern has been expressed regarding the delay of 24 to 48 hours required for bacteriologic culture, and clinicians often feel pressure to initiate therapy immediately, prior to obtaining culture results. Since treatment of streptococcal sore throat as long as 9 days after onset of symptoms is still effective in preventing rheumatic fever, initiation of antibiotics is rarely of urgent importance. Early antibiotic therapy may have beneficial effects in relieving symptoms and allowing an earlier return to school or daycare, but may have disadvantages. Some studies suggest that children receiving immediate antibiotic therapy are more likely to have symptomatic recurrences in the months following treatment than are children who delay the initiation of therapy by 48 hours. Delaying antibiotics may allow for an immune response to occur that protects the child against reinfection. These questions continue to be sources of controversy in the management of streptococcal pharyngitis.9-11
When the diagnosis of streptococcal pharyngitis seems particularly likely based on clinical findings, or an immediate decision about antibiotic therapy is required, the use of rapid antigen detection tests can be of value in confirming the diagnosis. Most rapid antigen detection kits use antibody for detection of the group A carbohydrate antigen in the cell wall. The specificity of these antibody tests is superior to their sensitivity. Currently available rapid streptococcal tests have a sensitivity of 70% to 90% compared with standard throat cultures. The sensitivity may depend on the inoculum obtained in the clinical sample: The sensitivity may be no greater than 75% for colonization (or light growth on culture) to approximately 95% in symptomatic pharyngitis (where culture usually reveals a heavy growth). When clinical suspicion or concern is high, a negative result with the rapid strep test should be confirmed by throat culture. When identified by culture, group A streptococcus does not require antibiotic susceptibility testing.12
Scarlet Fever
When a fine, diffuse erythematous rash (Fig. 285-1) is present in the setting of acute streptococcal pharyngitis, the illness is called scarlet fever. The modes of streptococcal transmission, age distribution, and other epidemiologic features are identical to those for streptococcal pharyngitis. The rash is caused by the pyrogenic exotoxins, SPE A, B, C, and F. It often is noticed initially on the neck and upper chest. It is a diffuse, finely papular, erythematous eruption producing a bright red discoloration of the skin, which blanches on pressure. The texture is that of fine sandpaper. The flexor skin creases, particularly in the antecubital fossae, and the groin area may be unusually prominent (Pastia’s lines). The circumoral skin is pale, giving the appearance of pallor. Small vesicular lesions (miliary sudamina) may appear on the abdomen, hands, and feet. Toward the end of the first week of illness, the rash fades, followed by desquamation over the trunk which progresses to the hands and feet. Scarlet fever may be confused with roseola, Kawasaki disease, drug eruptions, and toxin-mediated Staphylococcus aureus infections.
Streptococcal Skin Infections
The most common form of skin infection due to group A streptococcus is superficial pyoderma. Also referred to as streptococcal impetigo (or impetigo contagiosa), it occurs commonly in tropical climates, but can be highly prevalent in temperate climates as well, particularly during the summer months. Major risk factors that predispose to this infection include local injury to skin caused by insect bites, scabies, atopic dermatitis, and minor trauma. This form of streptococcal infection is usually painless and the patient is usually afebrile. Streptococcal impetigo usually has the highest incidence in young children (ages 2–5 years). Streptococcal impetigo usually appears first as a discrete papulovesicular lesion surrounded by a localized area of redness. The vesicles rapidly become purulent and then crusted, in contrast to the classic bullous appearance of impetigo resulting from S aureus. Lesions are most commonly encountered on the face and extremities. If untreated, streptococcal impetigo is usually a mild but chronic illness, often spreading to other parts of the body. Regional lymphadenitis may be observed. Pyoderma-associated group A strains such as M/emm 49, M/emm 55, and M/emm 57 are associated with poststreptococcal glomerulonephritis. The M/emm types that give rise to streptococcal tonsillitis (eg, types 1, 3, 4, 5, 6, 12) uncommonly cause streptococcal impetigo.
Deeper soft tissue infections may occur as the result of S pyogenes. A deeply ulcerated form of streptococcal impetigo, ecthyma, may complicate streptococcal impetigo. This form of infection tends to involve deeper layers of the skin and is encountered mainly in the tropics. Streptococcal cellulitis is an acute, rapidly spreading infection of skin and subcutaneous tissue that can follow burns, wounds, surgical incisions, varicella infection, and mild trauma. In contrast to impetigo, pain, tenderness, swelling and erythema, and systemic toxicity are common, and patients may have associated bacteremia. Prompt antibiotic therapy and careful serial examination are crucial, as cellulitis may progress to necrotizing fasciitis. Perianal cellulitis and vaginitis should be considered in children who complain of perineal discomfort or vaginal discharge. Erysipelas is now a relatively rare acute streptococcal infection involving the deeper layers of the skin and the underlying connective tissue. The face is a commonly involved site, especially in adults. The skin over the affected area is swollen, red, and very tender, and superficial blebs may be present. The most characteristic finding in erysipelas is the sharply defined, slightly elevated border, in contrast to the indistinct border of streptococcal cellulitis. Cultures obtained by leading edge needle aspiration of inflamed areas will often result in a positive culture.
Necrotizing Fasciitis
Necrotizing fasciitis resulting from S pyogenes (so-called streptococcal gangrene) is an acute, rapidly progressive, severe deep infection of the fascia and subcutaneous tissues and is associated with extensive destruction of superficial and deep fascia. Recent varicella infection has proven to be an important risk factor for necrotizing fasciitis, although routine childhood immunization against chickenpox has had an impact on the incidence of this complication.13 The onset is heralded by diffuse erythematous swelling, with exquisite pain at the affected site that typically seems incompatible with the degree of swelling noted by the clinician. As the lesion progresses, often quite rapidly, the skin becomes bluish gray. It is not uncommon for large hemorrhagic bullae to appear over the area. The area of involvement under the skin is usually much larger than seems evident from the superficial examination of skin. Compartment syndromes often occur. Repeated surgical debridement of necrotic tissue is crucial. Differentiating streptococcal cellulitis from necrotizing fasciitis can be difficult; therefore, careful frequent serial physical examinations are crucial. Surgical consultation early in the course of infection is essential.
Streptococcal Toxic Shock Syndrome
Streptococcal toxic shock syndrome (TSS) is characterized by hypotension and multiple organ failure.14 Criteria proposed by a CDC Working Group on Severe Streptococcal Infections for the diagnosis of streptococcal toxic shock are outlined in Table 285-1. There is considerable overlap with streptococcal necrotizing fasciitis; most cases occur in association with soft tissue infections. However, streptococcal TSS may occur in association with other focal streptococcal infections, including pharyngeal infection. Renal impairment occurs in approximately 80% of patients, and hepatic dysfunction occurs in 65%. ARDS is often present in severe cases and should be looked for. The pathogenesis of streptococcal toxic shock syndrome appears to be related in part to the ability of certain SPEs (A, C, and F) to function as superantigens.
Other Suppurative Complications
Suppurative complications resulting from the spread of streptococci to adjacent structures are occasionally observed. Cervical adenitis, peritonsillar abscess, retropharyngeal abscess, otitis media, mastoiditis, and sinusitis occur in children in whom the primary illness has gone unnoticed or in whom treatment of the pharyngitis has been incomplete. S pyogenes may cause pneumonia, parapneumonic effusion, and epiglottitis. Group A streptococcus is a common etiology of acute hematogenous osteomyelitis. Isolated bacteremia, meningitis, and endocarditis have been described, but these are relatively rare manifestations of group A streptococcal infection.
Post-Infectious Complications of Group A Streptococcus Infection
Acute rheumatic fever is discussed in detail in Chapter 235, where the Jones criteria for diagnosis are detailed. Only 25% to 40% ARF patients have a positive throat culture at the time of presentation. The most reliable evidence of an antecedent group A streptococcal infection is often identification of a serologic response to the organism.19
Glomerulonephritis can follow group A streptococcal infections of either the pharynx or the skin. Its occurrence requires the presence of so-called nephritogenic strains of group A streptococci in the community. Type 12 is one of the most common M/emm serotypes causing post-streptococcal glomerulonephritis after pharyngitis, and M/emm type 49 is a type commonly related to pyoderma-associated nephritis. The latent period between the group A streptococcal infection and the onset of glomerulonephritis varies from 1 to 2 weeks after streptococcal pharyngitis, but is approximately 21 days after pyoderma. Like ARF, the pathogenesis of PSGN appears to be immunologically mediated (see Chapter 472).
Sydenham chorea is the most common cause of acquired chorea in children and occurs most commonly between ages 5 and 15 years. It is a cardinal feature of rheumatic fever and is sufficient alone to make a diagnosis. SCH usually occurs several weeks to months after untreated streptococcal infection (beta hemolytic streptococcus) (see Chapter 566). In 1998 a syndrome known as pediatric autoimmune neuropsychiatric disease associated with streptococci (PANDAS) was described. Patients are usually prepubertal children who develop multiple neuropsychiatric symptoms (tics and obsessive-compulsive behavior), and it was suggested that this was temporally related to group A streptococcal infection. Whether or not this syndrome is related to streptococcal infection remains unconfirmed. There is no evidence from controlled studies to support the efficacy of continuous or suppressive antimicrobial therapy for these children. (See Chapter 566.)
Table 285-1. Definition of Streptococcal Toxic Shock



