Overview of steroid hormone biosynthesis
Four major sources of cholesterol are used for steroid hormone biosynthesis:
de novo cholesterol biosynthesis (from acetate)
plasma membrane
intracellular lipid droplets (containing cholesteryl esters, such as cholesteryl oleate)
plasma lipoproteins (primarily low-density lipoprotein cholesterol).
The rate-determining step in the synthesis of steroid hormones is the removal of the 6-carbon side chain from the 27-carbon substrate cholesterol to generate the first 21-carbon steroid, pregnenolone (Figure 6.2). This reaction is catalysed by the cytochrome P450 (CYP)-based cholesterol side chain cleavage enzyme (CYP11A1, previously named P450SCC), which is located in the inner mitochondrial membrane facing the matrix. The relevance of this fact is that the lipid substrate cholesterol has to be transported across the aqueous space that lies between the outer and the inner mitochondrial membranes. The intracellular trafficking of cholesterol to the CYP11A1 enzyme relies on the actions of:
steroidogenic acute regulator (StAR) protein
mitochondrial peripheral benzodiazepine receptors.
Cellular levels of StAR increase following stimulation of steroidogenic cells by luteinising hormone, follicle-stimulating hormone, human chorionic gonadotrophin or adrenocorticotrophic hormone. The StAR protein then acts as a ligand for the mitochondrial peripheral benzodiazepine receptors, which bring the mitochondrial membranes into apposition by activating voltage-dependent anion channels to mediate CI– efflux from the aqueous intermembrane space.
| Class of steroid | Number of carbon atoms | Major physiological example |
|---|---|---|
| CORTICOSTEROIDSa | ||
| Mineralocorticoids | 21 | Aldosterone |
| Glucocorticoids | 21 | Cortisol |
| Androgens | 19 | DHEA |
| GONADAL STEROIDS b | ||
| Progestogens | 21 | Progesterone |
| Androgens | 19 | Testosterone |
| Estrogens | 18 | Estradiol |
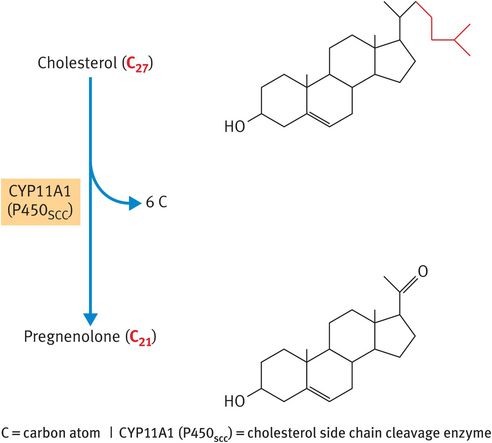
Metabolism of cholesterol to pregnenolone
The significance of StAR in steroidogenesis is underscored in pregnancies complicated by lipoid congenital adrenal hyperplasia. In this condition, failure of StAR in the fetal adrenal glands prevents transport of cholesterol into the mitochondria of the adrenocortical cells, leading to the accumulation of cholesteryl esters within engorged lipid droplets in the adrenal cortex. As a consequence, the fetal adrenal glands fail to make any of the corticosteroid hormones. This rare condition is usually lethal in utero. If affected infants are delivered, they have problems inflating their lungs (because of the absence of fetal glucocorticoids in utero) and enter a salt-wasting crisis (because of the absence of aldosterone).
Steroidogenic enzymes
Although the pathways for steroid biosynthesis seem to be complex, they revolve around just two classes of enzyme:
CYP enzymes
hydroxysteroid dehydrogenase (HSD) enzymes.
The function of the CYP enzymes is to catalyse the hydroxylation reactions required to increase the solubility of the steroid hormones in aqueous media (blood, extracellular fluid and urine). In addition, some of the CYP enzymes – specifically CYP11A1 and steroid 17α-hydroxylase (CYP17A or P450c17) – also act as lyase enzymes to cleave single carbon bonds.1 The hydroxylase function of CYP enzymes relies on the transport of electrons from the reduced form of the nucleotide co-factor nicotinamide adenine dinucleotide phosphate (NADPH) to the haem centre of the CYP enzyme via ferredoxin reductase and ferredoxin (Figure 6.3).
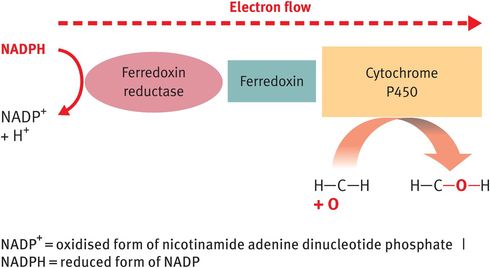
Operation of steroidogenic enzymes: cytochrome P450 enzymes
The HSD enzymes (3β-HSD and 17β-HSD) are oxidoreductase enzymes that belong to the superfamily of short-chain alcohol dehydrogenases. As such, the HSD enzymes catalyse the oxidation of secondary alcohol groups and/or the reduction of ketone groups on steroid hormones at designated carbon positions (Figure 6.4). In addition, 3β-HSD acts as a Δ5,Δ4 isomerase to convert the weak steroids pregnenolone and dehydroepiandrosterone to the more potent steroid metabolites progesterone and androstenedione, respectively. During this reaction, the double bond between carbon positions 5 and 6 (signified by the symbol Δ5) of the steroid B ring is relocated to carbon positions 4 and 5 (signified by the symbol Δ4) of the A ring (Figure 6.5A). By contrast, the 17β-HSD enzymes act primarily as reductase enzymes, reducing the ketone at carbon position 17 on androstenedione and estrone to the more potent beta-alcohol group at carbon position 17, characteristic of testosterone and estradiol (Figure 6.5B).
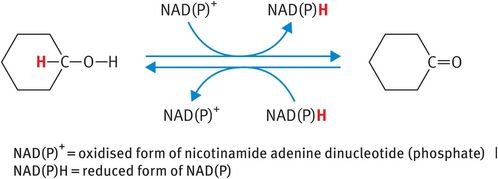
Operation of steroidogenic enzymes: hydroxysteroid dehydrogenase enzymes

Steroid metabolism by hydroxysteroid dehydrogenase enzymes
Tissue-specific patterns of steroidogenesis
The pathway of steroid biosynthesis within a given steroidogenic tissue depends on the precise pattern of CYP and HSD expression in that tissue:
In testis Leydig cells, CYP11A1, CYP17A, 3β-HSD and 17β-HSD act in sequence to synthesise testosterone (Figure 6.6A).
In the theca cells of ovarian follicles, CYP11A1, CYP17A and 3β-HSD act in sequence to synthesise androstenedione (Figure 6.6B).
In the follicular granulosa cells, follicle-stimulating hormone upregulates the enzyme aromatase (CYP19 or P450AROM), which converts androstenedione from the theca cells into estrone. Estrone can subsequently be reduced by 17β-HSD to the more potent estrogen, estradiol (Figure 6.6C).
In the steroidogenic cells of the corpus luteum, CYP11A1, 3β-HSD, CYP17A, CYP19 and 17β-HSD are all expressed, enabling the luteal cells to synthesise both progesterone and estradiol (Figure 6.6D).
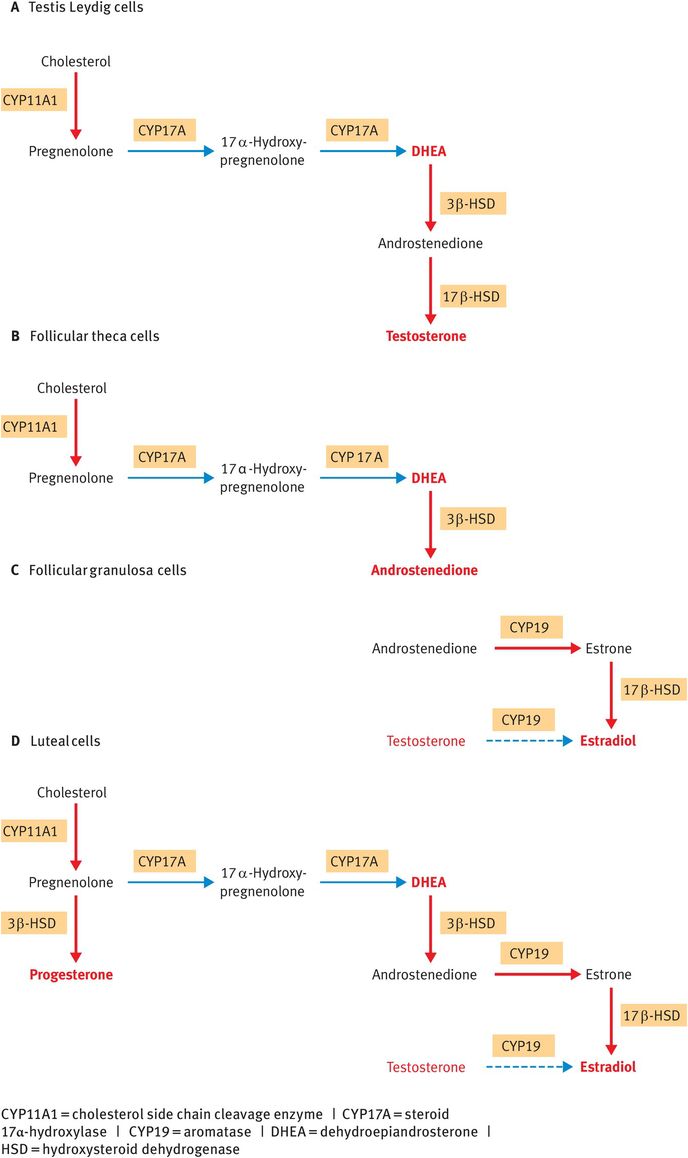
Tissue-specific steroidogenic pathways
The pattern of steroid synthesis in the placenta is complicated by the fact that the placenta is an ‘incomplete endocrine gland’: it lacks the key enzymes required to synthesise the full range of steroid hormones. Most notably, the placenta does not express the CYP17A enzyme necessary to convert progestogens to androgens. As a consequence, for the placenta to synthesise estrogens, it has to work in concert with the fetal adrenal gland and maternal liver, with steroids passing back and forth between the placenta, fetal and maternal circulations. Importantly, 16α-hydroxyandrostenedione secreted from the fetal zone of the fetal adrenal cortex can, in the placenta, be aromatised to estriol, a unique steroid that is synthesised only by the placenta and that can be used as a surrogate marker to assess the functional status of the fetal adrenal gland.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


