Background
Most small (birthweight <10%) for-gestational-age cases occur at term, in uncomplicated pregnancies, and are not identified during prenatal visits as having fetal growth restriction. Hence, they do not benefit from antepartum surveillance and timed delivery. There is dismissive and disquieting opinion that small for gestational age among uncomplicated pregnancies is not associated with increased morbidities and, therefore, does not warrant improved detection. Our hypothesis was that among uncomplicated pregnancies at term, small for gestational age have significantly higher morbidity and mortality than appropriate (birthweight 10-89%) for gestational age.
Objective
We sought to compare composite neonatal morbidity among uncomplicated term singleton pregnancies with small vs appropriate for gestational age.
Study Design
We culled collected data from 9 completed Maternal-Fetal Medicine Units studies conducted from 1989 through 2004. All data were collected prospectively by trained staff. We excluded women who delivered <37 weeks and those with hypertension or diabetes, multiple gestation, known anomalies, and birthweight of ≥90% for gestational age. Using multivariable analysis, we compared composite neonatal morbidity, which included stillbirth and neonatal mortality between small and appropriate for gestational age. Random effect logistic regressions were used to account for study heterogeneity, with adjustment for potential confounders. We calculated adjusted odds ratios and 95% confidence intervals.
Results
Of the >91,000 women enrolled in the studies, 60% (n = 50,011) met the inclusion criteria. Among the uncomplicated pregnancies, 10.8% (n = 5416) were small for gestational age. The rate of composite neonatal morbidity of 16% in small for gestational age and 10% in appropriate for gestational age persisted (adjusted odds ratio, 1.75; 95% confidence interval, 1.71–1.78). After adjustment for confounders, the following neonatal morbidities were significantly more common among term small than appropriate for gestational age: Apgar <4 at 5 minutes, respiratory distress syndrome, mechanical ventilation, necrotizing enterocolitis grade 2 or 3, and neonatal sepsis. Lastly, rate of stillbirths (3.5 vs 0.9/1000 births; adjusted odds ratio, 3.49; 95% confidence interval, 1.83–6.67) and neonatal mortality (1.1 vs 0.4/1000 births; adjusted odds ratio, 2.56; 95% confidence interval, 1.83–3.57) were significantly more common with small than appropriate for gestational age. In secondary analyses the composite neonatal morbidity among newborns at <5% and at 5-9% was significantly higher than appropriate for gestational age. Lastly, in subgroup analyses of women who delivered at 37.0-38.6 weeks or at ≥39.0 weeks, the increased rate of composite neonatal morbidity, stillbirth, and neonatal mortality among small for gestational age persisted.
Conclusion
Among uncomplicated pregnancies at term, small- compared to appropriate-for-gestational-age newborns have a significantly higher likelihood of composite neonatal morbidity, stillbirth, and neonatal mortality. A large multicenter trial is warranted to determine if improved detection of small for gestational age among uncomplicated pregnancies can mitigate morbidities and mortality, without disproportionate interventions and iatrogenic complications.
Introduction
Small-for-gestational-age (SGA) newborns, defined as birthweight <10%, are at increased risk of both neonatal morbidity (respiratory distress syndrome, intraventricular hemorrhage, seizure, sepsis) and mortality including risk of stillbirth and death within 28 days of birth. Frequently, the publications linking SGA with morbid sequelae include all pregnancies irrespective of maternal comorbidity, gestational age (GA) at birth, presence of premature rupture of membranes, hypertensive disease, pregestational diabetes, or multiple gestations. There is, however, a paucity of publications on neonatal morbidity and mortality among SGA at term (≥37 weeks) without concomitant maternal comorbidities such as hypertension or diabetes.
The importance of focusing on SGA among uncomplicated pregnancies is that the majority of newborns with weight <10th percentile are born at term. Lee et al, for example, reported that in 2010 of the 32 million SGA born in 138 developing countries, >29 million were born at term. In 2014, of the 3.98 million births in the United States, about two thirds of pregnancies were uncomplicated and, among them, the likelihood of SGA is roughly 10%. Thus, we estimate that annually in the United States, there are >235,000 SGA newborns born at term from uncomplicated pregnancies and there is a significant knowledge gap about their outcomes.
The primary purpose of this secondary analysis of 9 Maternal-Fetal Medicine Unit (MFMU) Network studies was to compare the morbidity and mortality among SGA vs appropriate for GA (AGA) (birthweight between 10-89% for GA) among term pregnancies without known comorbidities. The secondary purpose was to compare: (1) the adverse outcomes among infants with birthweight <5% and 5-9% to AGA newborns; and (2) the morbidity and mortality for the subgroups of deliveries occurring at 37.0-38.6 weeks (early term) and at ≥39.0 weeks (term).
Materials and Methods
All MFMU databases available to us as member of the network were considered for this study. These included: (1) a randomized trial of low-dose aspirin to prevent preeclampsia ; (2) a preterm prediction study ; (3) a clinical trial of low-dose aspirin to prevent preeclampsia in high-risk women ; (4) a randomized trial of 17-alpha hydroxyprogesterone caproate for the prevention of preterm birth in high-risk women ; (5) an observational study of cesarean delivery and vaginal birth after cesarean delivery ; (6) a randomized clinical trial of the beneficial effects of antenatal magnesium sulfate ; (7) a randomized placebo-controlled trial of antenatal corticosteroids regimens ; (8) a prospective observational study of effects of factor V Leiden mutation on maternal and perinatal outcomes ; and (9) midtrimester endovaginal sonography in women at high-risk for spontaneous preterm birth. The obstetrical determinant of neonatal survival database was not included due to the limited number of eligible participants and obstetrical variables collected. Full details of these studies have been previously reported.
All variables and data from these studies were collected prospectively by trained research nurses following strict and specific protocols outlined in the manual of operations. All eligible databases were combined, all variables of interest were aligned, and definitions standardized using the original designations within each study’s protocol. We used uniform coding for all study variables.
The inclusion criteria for our study were nonanomalous singleton pregnancies, with a documented estimated GA (EGA) and birthweight, a GA ≥37.0 weeks at birth, and birthweight <90% for GA. Methodology utilized to determine GA for each trial included was previously reported. For this analysis, birthweight percentiles were calculated using the data reported by Alexander et al. This reference curve was used as it was determined to be the most robust and most contemporary of the growth curves available. SGA included all birthweights <10th percentile while AGA was birthweights between 10th-89th percentile.
We excluded women who had a multiple gestation, anomalies, EGA <37 weeks, medical or obstetrical complications like pregestational or gestational diabetes, hypertensive disease of pregnancy, chronic hypertension, missing EGA or birthweight, and potential duplicates, ie, women who may have potentially participated in >1 study during the same pregnancy. A mother was considered a duplicate if she matched all of the following variables across ≥2 studies: maternal age, parity, GA at delivery, route of delivery, neonatal gender, birthweight, and Apgar score at both 1 and 5 minutes.
Composite neonatal morbidity (CNM) was defined as any of the following: Apgar score <4 at 5 minutes, respiratory distress syndrome, need for mechanical ventilation, intraventricular hemorrhage grade III or IV, necrotizing enterocolitis stage 2 or 3, neonatal sepsis–suspected or proven, confirmed seizure, and stillbirth or neonatal death. Each parameter of CNM was previously defined in the parent publications of the 9 trials. Stillbirth was defined as any fetal death occurring prior to or during labor and neonatal mortality as death occurring after delivery up to 28 days after birth.
The sample size for this analysis was determined by the size of all databases included. Descriptive statistics were used to report all variables of interest. Crude odds ratios (OR) and 95% confidence intervals (CI) from random effect logistic regression models were utilized. We fit separate models to each component of CNM and to the composite primary outcome. Mixed effect logistic regressions with a random intercept for study (to account for study heterogeneity) were adjusted for maternal age (<20, 20-34, ≥35 years), race/ethnicity (Caucasian, Afro-American, Hispanic, other), marital status (yes, no), education level (≤12 or >12 years of education), nulliparity (yes, no), prepregnancy body mass index (BMI) (<30, ≥30 kg/m 2 ), maternal smoking (yes, no), maternal alcohol use (yes, no), neonatal gender (male, female), and diagnosis of chorioamnionitis (yes, no). In cases where the logistic mixed effects model did not converge, we used logistic regression with robust SE accounting for the cluster study effect and adjusted for the same covariates. Secondary analyses used similar models to compare neonatal outcomes in infants with birthweight <5% and those at 5-9% to AGA newborns.
We imputed missing data in BMI and education variables (24% and 27% missing, respectively) using the method of multivariate imputation by chained equation. Variables included in the imputation model were the same as those included in the analyses models. We generated 10 imputed data sets and combined coefficient estimates across these using Rubin rules. Adjusted OR (aOR) and 95% CI from multiple imputation estimates are presented. A P value <.05 was considered statistically significant.
To assess the influence of GA and morbidity, we ran subgroup analyses on all outcomes of interest for infants delivered at 37.0-38.6 weeks (early term pregnancy) and those delivered at ≥39 weeks (full-term pregnancy). All analyses were performed in software (Stata v13; StataCorp LP, College Station, TX). The STrengthening the Reporting of OBservational studies in Epidemiology guidelines for reporting observational studies were followed. This analysis qualified for exempt status from the institutional review board at the University of Texas Health Science Center at Houston because it involved the study of publically available deidentified data.
Materials and Methods
All MFMU databases available to us as member of the network were considered for this study. These included: (1) a randomized trial of low-dose aspirin to prevent preeclampsia ; (2) a preterm prediction study ; (3) a clinical trial of low-dose aspirin to prevent preeclampsia in high-risk women ; (4) a randomized trial of 17-alpha hydroxyprogesterone caproate for the prevention of preterm birth in high-risk women ; (5) an observational study of cesarean delivery and vaginal birth after cesarean delivery ; (6) a randomized clinical trial of the beneficial effects of antenatal magnesium sulfate ; (7) a randomized placebo-controlled trial of antenatal corticosteroids regimens ; (8) a prospective observational study of effects of factor V Leiden mutation on maternal and perinatal outcomes ; and (9) midtrimester endovaginal sonography in women at high-risk for spontaneous preterm birth. The obstetrical determinant of neonatal survival database was not included due to the limited number of eligible participants and obstetrical variables collected. Full details of these studies have been previously reported.
All variables and data from these studies were collected prospectively by trained research nurses following strict and specific protocols outlined in the manual of operations. All eligible databases were combined, all variables of interest were aligned, and definitions standardized using the original designations within each study’s protocol. We used uniform coding for all study variables.
The inclusion criteria for our study were nonanomalous singleton pregnancies, with a documented estimated GA (EGA) and birthweight, a GA ≥37.0 weeks at birth, and birthweight <90% for GA. Methodology utilized to determine GA for each trial included was previously reported. For this analysis, birthweight percentiles were calculated using the data reported by Alexander et al. This reference curve was used as it was determined to be the most robust and most contemporary of the growth curves available. SGA included all birthweights <10th percentile while AGA was birthweights between 10th-89th percentile.
We excluded women who had a multiple gestation, anomalies, EGA <37 weeks, medical or obstetrical complications like pregestational or gestational diabetes, hypertensive disease of pregnancy, chronic hypertension, missing EGA or birthweight, and potential duplicates, ie, women who may have potentially participated in >1 study during the same pregnancy. A mother was considered a duplicate if she matched all of the following variables across ≥2 studies: maternal age, parity, GA at delivery, route of delivery, neonatal gender, birthweight, and Apgar score at both 1 and 5 minutes.
Composite neonatal morbidity (CNM) was defined as any of the following: Apgar score <4 at 5 minutes, respiratory distress syndrome, need for mechanical ventilation, intraventricular hemorrhage grade III or IV, necrotizing enterocolitis stage 2 or 3, neonatal sepsis–suspected or proven, confirmed seizure, and stillbirth or neonatal death. Each parameter of CNM was previously defined in the parent publications of the 9 trials. Stillbirth was defined as any fetal death occurring prior to or during labor and neonatal mortality as death occurring after delivery up to 28 days after birth.
The sample size for this analysis was determined by the size of all databases included. Descriptive statistics were used to report all variables of interest. Crude odds ratios (OR) and 95% confidence intervals (CI) from random effect logistic regression models were utilized. We fit separate models to each component of CNM and to the composite primary outcome. Mixed effect logistic regressions with a random intercept for study (to account for study heterogeneity) were adjusted for maternal age (<20, 20-34, ≥35 years), race/ethnicity (Caucasian, Afro-American, Hispanic, other), marital status (yes, no), education level (≤12 or >12 years of education), nulliparity (yes, no), prepregnancy body mass index (BMI) (<30, ≥30 kg/m 2 ), maternal smoking (yes, no), maternal alcohol use (yes, no), neonatal gender (male, female), and diagnosis of chorioamnionitis (yes, no). In cases where the logistic mixed effects model did not converge, we used logistic regression with robust SE accounting for the cluster study effect and adjusted for the same covariates. Secondary analyses used similar models to compare neonatal outcomes in infants with birthweight <5% and those at 5-9% to AGA newborns.
We imputed missing data in BMI and education variables (24% and 27% missing, respectively) using the method of multivariate imputation by chained equation. Variables included in the imputation model were the same as those included in the analyses models. We generated 10 imputed data sets and combined coefficient estimates across these using Rubin rules. Adjusted OR (aOR) and 95% CI from multiple imputation estimates are presented. A P value <.05 was considered statistically significant.
To assess the influence of GA and morbidity, we ran subgroup analyses on all outcomes of interest for infants delivered at 37.0-38.6 weeks (early term pregnancy) and those delivered at ≥39 weeks (full-term pregnancy). All analyses were performed in software (Stata v13; StataCorp LP, College Station, TX). The STrengthening the Reporting of OBservational studies in Epidemiology guidelines for reporting observational studies were followed. This analysis qualified for exempt status from the institutional review board at the University of Texas Health Science Center at Houston because it involved the study of publically available deidentified data.
Results
From 1989 through 2004, in the 9 MFMU studies, 91,373 women were enrolled of whom 83,815 (92%) had singleton pregnancies. Among these women, we excluded 33,804 (40.3%) for the following reasons: 32,005 were less than 37 weeks at birth, were missing birth weight or gestational age at birth or were labeled as large for gestational age (LGA); 1661 were anomalous, and 138 patients were excluded as suspected duplicates. For our analyses, 50,011 (54.7%) singletons met the inclusion criteria and are the focus of our report. The prevalence of SGA in our cohort was 10.8% ( Figure 1 ).
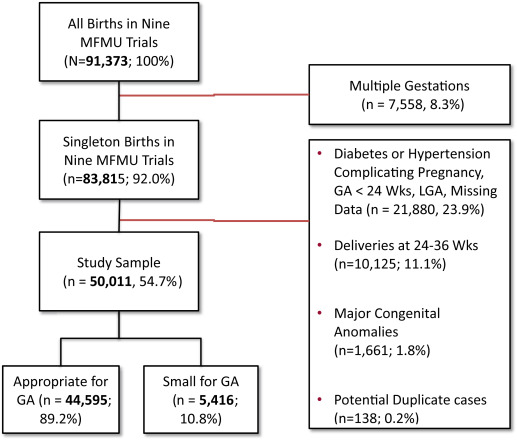
Baseline characteristics differed significantly between SGA and AGA groups for maternal age; ethnicity; nulliparity; education; marital status; self-reported cigarette, alcohol, or drug use; and BMI at delivery. The 3 variables associated with significantly lower likelihood of SGA were having at least high school education, being married, and at delivery having BMI of ≥30 kg/m 2 ( Table 1 ).
| SGA, N = 5416 | AGA, N = 44,595 | OR a (95% CI) | |
|---|---|---|---|
| Maternal age, y | |||
| <20 | 3952 (73.0) | 33,530 (75.2) | 1 |
| 20–34 | 750 (13.8) | 4537 (10.2) | 1.30 (1.19–1.42) b |
| ≥35 | 714 (13.2) | 6526 (14.6) | 0.95 (0.87–1.03) |
| Ethnicity | |||
| Caucasian | 1463 (27.0) | 16,842 (37.7) | 1 |
| Afro-American | 2569 (47.5) | 13,626 (30.6) | 2.11 (1.97–2.26) b |
| Hispanic | 1167 (21.5) | 12,138 (27.2) | 1.11 (1.03–1.20) b |
| Other | 217 (4.0) | 1989 (4.5) | 1.28 (1.10–1.48) b |
| Nulliparous | 1401 (26.0) | 10,225 (23.0) | 1.14 (1.06–1.22) b |
| High school education | 1041 (26.2) | 11,639 (35.5) | 0.67 (0.62–0.72) c |
| Married | 2384 (44.0) | 25,917 (58.1) | 0.55 (0.52–0.58) c |
| Smoker | 1496 (27.7) | 6101 (13.7) | 2.38 (2.22–2.54) b |
| Alcohol | 478 (9.6) | 2494 (6.0) | 1.54 (1.36–1.74) b |
| Drugs | 465 (10.0) | 1526 (3.9) | 2.59 (2.31–2.90) b |
| BMI at delivery, km/m 2 | |||
| <30 | 3086 (80.1) | 26,025 (76.3) | 1 c |
| ≥30 | 764 (19.9) | 8054 (23.7) | 0.82 (0.75–0.89) c |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


