Fig. 15.1
Lymph node evaluation road map. SLN, sentinel lymph node; LND, lymphadenectomy
Sentinel Lymph Nodes and Endometrial Cancer
The first reported use of SLN mapping in endometrial cancer was described by Burke and colleagues in a 1996 pilot study [14]. Numerous studies have followed with varying methodologies for SLN detection. In these studies, the major distinctions are with respect to the type of detection dye used and the location of the injection site (Fig. 15.2).
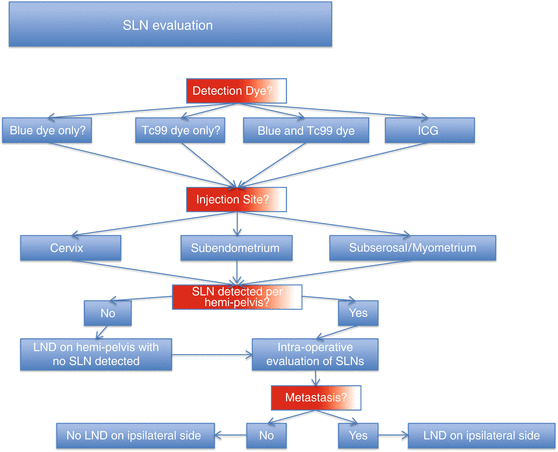

Fig. 15.2
Sentinel lymph node evaluation decision tree. SLN, sentinel lymph node; Tc99, technetium-99 sulfur colloid; ICG, indocyanine green dye; LND, lymphadenectomy
Detection Methods
In the majority of cases, detection of SLNs is done via visualization of colored lymphatics leading to colored nodes (colorimetric detection), detection of radioactivity in the nodes (isotopic detection), or both.
“Blue Dye” Detection
Various blue dyes have been reported in literature and include isosulfan blue, patent blue, and methylene blue. There is no statistically significant difference in the detection rate among the different blue dyes. They will be referred to collectively as “blue dye” in this chapter. Though the injection site may vary, the injection of blue dye always occurs intraoperatively, following induction of anesthesia. After approximately a 10-min delay to allow diffusion of the blue dye, one can visualize the SLN by following blue lymphatic channels to an area of blue collection. The injection of blue dye is deemed safe, but rarely, adverse reactions are reported (e.g., allergic reaction mainly with methylene blue).
Isotope Detection
The patient usually receives a preoperative injection of technetium-99 sulfur colloid on the day prior to or on the morning of surgical staging. Frequently, a lymphoscintigraphy is obtained in order to grossly orient the surgeon to the SLN’s location. During the operation, the surgeon utilizes a handheld gamma probe to detect radioactivity. SLNs are identified through the gamma probe by having a tenfold greater radioactive count compared to background radiation. Of note, there is a correlation between the SLNs located via lymphoscintigraphy and during surgical SLN mapping. Ballester and colleagues found 68 % mapping by lymphoscintigraphy vs. 82 % by intraoperative mapping [15]. Improvements are obtained by decreasing the time delay between lymphoscintigraphy and surgical SLN detection [16].
Overall, a combination of both blue dye and technetium produces the highest detection rate and lowest false-negative rate [17] when compared to each individually. It is recommended that surgeons utilize both until technical competency is attained, especially when utilizing blue dye independently. Based on the experience from Abu-Rustum and colleagues, the learning curve for SLN biopsy is approximately 30 cases [18].
Immunofluorescent Detection
Recently, immunofluorescent imaging has been introduced for SLN detection. Rossi and colleagues used indocyanine green (ICG) fluorescent dye injected into the cervix at the 3 o’clock and the 9 o’clock positions in 20 patients [19]. The group utilized the fluorescent imaging mode on the robotic platform of the da Vinci Surgical System in order to visualize the fluorescent green lymphatics and demonstrated a SLN detection rate of 88 % and bilateral detection rate of 60 %. The minimum cervical injection dosage was determined to be 1 mg. Holloway and colleagues utilized a cervical injection of both blue and ICG dye in 35 patients [20]. Utilizing fluorescent imaging, they demonstrated a 97 % bilateral SLN detection rate (100 % bilateral detection rate when using both colorimetric and fluorescent imaging). In both studies, no adverse reactions were noted after ICG injection.
Injection Technique
Three main sites of injection have been utilized for SLN mapping: (1) subserosal/myometrium, (2) endometrium (via hysteroscopy), and (3) cervix.
Subserosal/Myometrial Injection
Subserosal injection was the first technique reported by Burke et al. [14]. In this study, a midline incision was performed, followed by pelvic washings for cytologic analysis and occlusion of the fallopian tubes with hemoclips bilaterally. Next, using 1 ml of 1 % blue dye per syringe (total of 3 syringes), they injected into three uterine locations subserosally: (1) most superior portion of the fundus and on the (2) ventral and (3) dorsal midline 2 cm below the superior injection site. Several studies have replicated this technique [14, 21–23]. Others have followed a similar approach but increased the number of subserosal injection sites [24, 25] and showed an increase in detection rate [24, 25]. Intraoperative injections of technetium at the three previously mentioned midline sites have also been used in association with a handheld gamma probe to identify radioactive nodes.
SLN detection rate via this approach has been variable from 45 % to 92 % (excluding one small study of eight patients who did not have any SLN detected) [26] (Table 15.1). Given the small size of studies reported in the literature (largest study having 67 patients) [29], it is difficult to determine the true detection rate of the subserosal approach. Recommendations based on a 2011 meta-analysis [17] are that exclusive subserosal injection should be avoided because it was associated with decreased sensitivity of SLN mapping to detect malignancy.
Table 15.1
Studies using subserosal myometrial injection
Study | N | Surgerya | Dyeb | Number of injection sites | Detection rate | Bilateral detection rate | Mean SLN per patient | Sensitivity | NPV | FNR |
|---|---|---|---|---|---|---|---|---|---|---|
Burke et al. [14] | 15 | 1 | B | 3 | 67 % | NR | 3.1 | NR | NR | NR |
Echt et al. [26] | 8 | 1 | B | 3 | 0 | 0 | 0 | NR | NR | NR |
Holub et al. [27]c | 13 | 2 | B | 3 | 62 % | NR | 1.15 | NR | NR | NR |
Gien et al. [28]c | 9 | 1 | B | 1 | 56 % | NR | NR | NR | NR | NR |
Li et al. [24] | 20 | 1 | B | 5 | 75 % | 73 % | 4.7 | 100 % | 100 % | 0 % |
Frumovitz et al. [21] | 18 | 1 | B, R | 3 | 45 % | 39 % | 1.6 | NR | NR | NR |
Altgassen et al. [25] | 23 | 1 | B | 8 | 92 % | NR | 3 | 63 % | 93 % | 37 % |
Lopes et al. [22] | 40 | 1 | B | 3 | 78 % | NR | 2.0 | 80 % | 96 % | 20 % |
Robova et al. [29]c | 67 | 1 | B, R | 1 | 73 % | 67 % | 2.2 | 100 % | 100 % | 0 % |
Of note, a new approach also injecting into the myometrium was reported in a 2013 study by Torné et al. [30]. In their study, transvaginal ultrasonography was utilized in order to identify the uterine tumor and was followed by passing a needle through the anterior vaginal fornix into the anterior uterine wall where 4 ml of technetium was injected into the outer two thirds of the myometrium. A second injection (4 ml of technetium) was given following passing the needle through the endometrial cavity and into the outer two thirds of the myometrium of the posterior wall of the uterus. SLNs were visualized via lymphoscintigraphy and an intraoperative gamma probe. In a cohort of 74 patients, the rate of SLN detection was 74 %. Para-aortic SLNs were detected in 45 % of patients. Sensitivity was 92 %, and negative predictive value was 97.7 %. Disadvantages to this procedure include the technical skill required to ensure adequate injection (without spilling dye into the peritoneal cavity) and detection failure that is associated with tumor size (sevenfold failure rate when tumors were at least 4 cm in size).
Hysteroscopic Endometrial Injection
Hysteroscopy has been utilized in order to inject into the tumor from the cavity. Visualizing the tumor via hysteroscopy, the surgeon injects the traceable dye around the tumor. If the tumor is focal, then it is injected peri-tumorally (2–3 mm away) in four quadrants (3, 6, 9, and 12 o’clock position) into the endometrium. For multiple or diffuse tumors, the dye was injected in five sites: fundus, right mid-lateral wall, left mid-lateral wall, the mid-anterior wall, or mid-posterior wall [28, 31–33]. SLN detection rates range from 33 % to 100 % (excluding a small study of three patients where no SLN was detected) [17] (Table 15.2). Again, this wide range of variability is due to significant influence of the small study effect. Advocates of the hysteroscopic method state that the approach allows for adequate mapping of the para-aortic area; this is aptly demonstrated by a recent study on a cohort of 59 patients [16]. Utilizing only technetium dye, they found a 95 % SLN detection rate with 56 % of patients having a SLN in the para-aortic area. Solima et al. attributed their high SLN detection rate due to the short interval between hysteroscopic injection and SLN detection (approximately 6 h).
Table 15.2
Studies using hysteroscopic injection
Study | N | Surgerya | Dyeb | Detection rate | Bilateral detection rate | Mean SLN per patient | Sensitivity | NPV | FNR |
|---|---|---|---|---|---|---|---|---|---|
Niikura et al. [31] | 28 | 1 | R | 82 % | 50 % | 3.1 | 100 % | 100 % | 0 % |
Fersis et al. [34] | 10 | 1 | R | 70 % | 20 % | 1.7 | 100 % | 100 % | 0 % |
Raspagliesi et al. [35] | 18 | 2 | B, R | 94 % | 56 % | 3 | NR | NR | NR |
Maccauro et al. [32] | 26 | 2 | B, R | 100 % | 18 % | 2.5 | 100 % | 100 % | 0 % |
Gien et al. [28]c | 3 | 1 | B | 0 % | 0 % | 0 % | NR | NR | NR |
Delaloye et al. [33] | 60 | 1 + 2 | B, R | 50 % | 45 % | 3.7 | 89 % | 98 % | 11 % |
Perrone et al. [36] | 17 | 1 | R | 65 % | 27 % | 1.3 | 100 % | 100 % | 0 % |
Feranec et al. [37] | 21 | 1 | B, R | 81 % | NR | 2 | 100 % | 100 % | 0 % |
Robova et al. [29]c | 24 | 1 | B, R | 50 % | NR | 2.2 | 100 % | 100 % | 0 % |
Rossi et al. [38]c | 12 | 3 | G | 33 % | 50 % | 2.5 | NR | NR | NR |
In theory, a peri-tumoral injection is most likely to mimic the natural lymphatic drainage of malignant cells. However, the approach does have several drawbacks secondary to utilizing hysteroscopy. For example, given the increased time elapsed from time of injection until access to the abdominal cavity, lower detection rates were seen when using exclusively blue dye [31]. Hysteroscopy is technically challenging and not only prolongs operative time but also may be less reproducible among practitioners compared to other methods. Finally, there is an increased risk of iatrogenic dissemination of malignant cells. Maccauro et al. and Raspagliesi et al. each reported one case of positive peritoneal cytology following hysteroscopic injection [32, 35]. The significance of this positive cytology is controversial, and the risk appears to be reduced if the endometrial pressures remain below 70 mm Hg [39].
Cervical Injection
Most studies evaluating SLN mapping in endometrial cancer have utilized the cervix as the site of injection. For the cervical approach, many of the studies in the literature report the use of both technetium and blue dye [17, 40–42]. In most studies, the technetium is injected preoperatively, and the blue dye is injected in the operating room. Cervical injection sites vary from two (3 and 9 o’clock) to four (3, 6, 9, and 12 o’clock) positions. The blue tracer is injected in the cervix at the 3 o’clock and 9 o’clock positions in the operating room, following general anesthesia immediately prior to the surgical incision. At each cervical injection site, two 25-gauge needles are used to inject superficially (2–3 mm) into the submucosa as well as deeply (1–2 cm) toward the lower uterine segment [40, 41, 43]. Using this approach, SLN detection rates have ranged from 62 % to 100 % [44] with detection rates ranging from 84 % to 92 % for studies with the largest sample populations [40, 41, 45, 46] (Table 15.3).
Table 15.3




Studies using cervical injection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


