Salmonella, Shigella, and Escherichia coli
Andrew T. Pavia
The family Enterobacteriaceae is a large, heterogeneous group of gram-negative bacteria. Many are normal inhabitants of the gastrointestinal tract of humans and other animals, but members also frequently cause disease in human beings.
Among the Enterobacteriaceae, Salmonella, Shigella, Yersinia, and a number of specific phenotypes of Escherichia coli are important causes of gastroenteritis. In addition to diarrhea, these organisms cause a variety of extraintestinal infections. Each genera includes a heterogenous group of organisms that vary in their epidemiology and clinical characteristics. Enterobacteriaceae possess 3 major antigenic groups that react with antisera: (1) the O or somatic antigens, (2) the H or flagellar antigens, and (3) the K or capsular antigens.  This chapter discusses Salmonella, Shigella, and the diarrhea-causing E coli; Yersinia is discussed in Chapter 293.
This chapter discusses Salmonella, Shigella, and the diarrhea-causing E coli; Yersinia is discussed in Chapter 293.
SALMONELLA
Salmonella are gram-negative, aerobic, nonlactose-fermenting, nonsporulating, flagellated bacilli. Salmonella are considered a single species, but are divided into approximately 2500 serotypes based on the somatic antigen (the major determinant) plus 1 or more less-strongly reacting minor somatic antigens.1 Serotyping is performed by state health department laboratories after initial isolation of the organism. Serotyping is extraordinarily useful for epidemiologic purposes, but not necessary for initial clinical management. 
 EPIDEMIOLOGY AND PATHOPHYSIOLOGY
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Reptiles, birds, poultry, cattle, and pigs serve as the major reservoirs for nontyphoidal Salmonella. In contrast, human beings are the only reservoir for S typhi.
In the United States, the highest incidence of nontyphoid Salmonella infection is in the first year of life—greater than 110 laboratory-confirmed cases per 100,000 population per year (eFigs. 283.1 and 283.2  ).3, 4 Thereafter, rates of isolation decline rapidly by age 5 years and remain constant throughout adulthood. Salmonella infections show a seasonal pattern, with a consistent peak in the summer and fall. S typhi infection in the United States is uncommon (approximately 400 patients per year) and rarely occurs in children younger than 1 year of age. However, typhoid fever remains an important problem in many developing countries. In the United States, two thirds of S typhi infections are related to foreign travel.
).3, 4 Thereafter, rates of isolation decline rapidly by age 5 years and remain constant throughout adulthood. Salmonella infections show a seasonal pattern, with a consistent peak in the summer and fall. S typhi infection in the United States is uncommon (approximately 400 patients per year) and rarely occurs in children younger than 1 year of age. However, typhoid fever remains an important problem in many developing countries. In the United States, two thirds of S typhi infections are related to foreign travel.
Salmonella infections are acquired through ingesting the organism, most often from food, but waterborne, person-to-person, and animal-to-person transmission can occur. Important common source outbreaks have occurred as a consequence of contaminated milk, cheese, shell eggs, ice cream, and roast beef.
Iguanas and other pet reptiles and amphibians have emerged as an important source of infection for young children.5
Sentinel surveillance demonstrates that Salmonella are becoming increasingly resistant to ampicillin, chloramphenicol, streptomycin, tetracycline, kanamycin, cephalosporins, and aminoglycosides.6,7 Substantial evidence points to antibiotic use in animal husbandry as a major source of antibiotic resistance in human salmonellosis.
The dose necessary to cause clinical infection is estimated to be 105 to 1010 organisms based on volunteer studies in adults.8 However, the number of organisms necessary to cause illness is substantially lower in other situations, including more virulent organisms, low gastric acidity, prior use of antibiotics, in infants, in the elderly, and in those with defective cell-mediated immunity. Because of the relatively large number of organisms necessary to produce disease, water and person-to-person spread are less frequent sources of infection than is food, which can support multiplication of the organism. Contaminated water may play a larger role in typhoid fever. Spread of Salmonella in childcare centers is unusual.
Following infection, nontyphoid Salmonella are excreted in feces for a median of 5 weeks.9 In children younger than 5 years of age, 2.6% continue to excrete nontyphoid Salmonella beyond 1 year, compared with fewer than 1% of patients over 5 years old and older. The rate of carriage after infection is higher still in very young infants. In adults, 2% to 4% of those infected with S typhi become chronic carriers, often excreting the organism for the remainder of their lives. Long-standing infection of the gallbladder plays a role in chronic carriage. Despite the large number of chronic excretors of nontyphoid Salmonella, carriers are rarely implicated in outbreaks or sporadic disease. In contrast, chronic carriers play a pivotal role in typhoid fever.
The source of Salmonella for infants is less well understood. Chronic or transient asymptomatic carriage by the mother and cross-contamination during food preparation are probably important. Breast feeding is protective and exposure to reptiles and riding in a shopping cart next to poultry products are associated with infection.10 Nosocomial spread of disease has been documented by contaminated medical devices such as rectal thermometers, suction equipment, and baths.
There are several distinctive steps in Salmonella infection.11-15 Upon reaching the small intestine, the bacteria must attach to the epithelium. Salmonella invade M cells (mucosal antigen-presenting immune cells) and nonphagocytic epithelial cells. Within macrophages, they may not only survive but multiply. The ability of strains to survive and reproduce in macrophages is correlated with virulence in animal models. Diarrhea is probably induced by local inflammation, induction of inflammatory mediators, and in some strains, by 1 or more enterotoxins or cytotoxins. When examined histologically, the organism is prominent in Peyer patches. In some cases of nontyphoid salmonellosis, and in all cases of typhoid, the organisms reach the regional lymphatics. Bacteremia may result.
There are several important barriers to infection. The organism must survive gastric acid, which can rapidly kill Salmonella. Reduced gastric acidity as a result of extremes of age, medications, surgery, Helicobacter pylori infection, and foods that buffer gastric acid, increases the number of organisms that reach the small intestine. Normal intestinal flora are an important barrier. Prior treatment with antibiotics, particularly those that disrupt the predominant intestinal flora, increases the risk of infection with both antibiotic-resistant and antibiotic-sensitive strains.5,16,17 Cell-mediated immunity appears to be the primary immunologic defense against Salmonella infections. Susceptibility appears to be highest in the first few months of life, reflecting the developing immune system and low gastric acidity. Children with HIV infection, transplant recipients, and others on immunosuppressive agents, and children with advanced malignancies are at increased risk.18,19 Reticuloendothelial dysfunction is also associated with increased risk of Salmonella infection, including sickle cell disease, hemolytic anemias, and malaria.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The range of clinical manifestations of Salmonella infection includes asymptomatic infection, gastroenteritis, bacteremia, focal infection, urinary tract infection, and enteric fever. These symptom complexes may overlap. The incubation period for gastroenteritis is 6 to 72 hours (mean 36 hours); for enteric fever it is 7 to 21 days (usually 7–14 days). The incubation period is affected by the inoculum size.
Gastroenteritis
Diarrhea is the most common manifestation of salmonellosis. The diarrhea may be profuse and watery, reflecting predominant small-bowel involvement, or may involve smaller volume stools associated with mucus and fecal leukocytes, reflecting colonic involvement. Bloody diarrhea occurs in approximately 25% of cases. Fever, when present, tends to be highest within the initial day of onset. Headache, chills, anorexia, nausea, vomiting, and malaise may be present. Symptoms usually last 2 to 5 days, although diarrhea may be prolonged.
The most common complication of Salmonella gastroenteritis is dehydration and metabolic acidosis, occasionally progressing to hypovolemic shock. Infected children also may have concurrent bacteremia, may develop prolonged secretory diarrhea, or may manifest failure to thrive after acute infection.
Bacteremia
Bacteremia may occur during acute Salmonella gastroenteritis. Factors that increase the risk include age, underlying systemic illness, hemoglobinopathy, immunosuppression, and serotype of the infecting organism.20-22
In children with uncomplicated gastroenteritis, “silent” bacteremia occurs in 5% to 10%.21,23 The risk of bacteremia is markedly increased in infants less than 3 months of age, most likely because of the immaturity of their cell-mediated immune response. Other conditions associated with increased risk of bacteremia include malnutrition; hemolytic anemias, especially sickle cell disease; collagen vascular disease; schistosomiasis; bartonellosis; hematogenous or gastrointestinal tract malignancy; diabetes mellitus; previous therapy with antimicrobial agents; corticosteroids; and HIV infection. Salmonella bacteremia is common among adults and children with AIDS. Frequently, Salmonella bacteremia in AIDS patients presents with fever and a paucity of gastrointestinal symptoms. The illness is often prolonged, and relapses after therapy are common. After relapse, lifetime secondary prophylaxis may be necessary.
Focal Infection
Focal suppurative infections occur in about 10% of patients with bacteremia. Salmonella bacteremia can result in suppurative complication of almost any organ or tissue.24,25 Endocarditis most often involves abnormal or prosthetic valves, but infection of normal valves can occur. S choleraesuis has a propensity for causing endovascular infections. Osteomyelitis caused by Salmonella tends to occur in injured or infarcted bone. This probably explains the predisposition of children with hemoglobinopathies, especially sickle cell disease, to develop this complication.
Enteric Fever (Including Typhoid Fever)
The prototypic enteric fever is typhoid fever caused by S typhi, although clinically indistinguishable enteric fever has been reported with infections by S paratyphi A, S schottmuelleri, S hirschfeldii, and S choleraesuis and less commonly by other serotypes. The enteric fever caused by nontyphoid Salmonella generally has less morbidity than typhoid.
An average of 245 S typhi isolates are reported yearly to the CDC.26,27 Three quarters of patients report international travel within the 30 days before onset of illness. The onset of enteric fever is insidious in contrast to bacteremia due to other gram-negative bacteria.28,29 The number of bacteria ingested influence attack rate and the length of the incubation period. The initial signs of infection are malaise, anorexia, headache, myalgias, and fever. The fever begins insidiously, is hectic, and gradually rises over the initial week to as high as 40°C (104°F). A relative bradycardia disproportionate to the temperature elevation is characteristic. Although diarrhea may be present during the initial stages, constipation becomes a more prominent symptom as the illness progresses. Hepatomegaly and splenomegaly, often with diffuse abdominal tenderness, are common. The abdomen may be mildly tender; but marked distension, dilated loops, or significant tenderness may indicate ileus. Leukopenia is not uncommon.
Rose spots occur in a small proportion of patients toward the second week. They are discrete (2–4 mm), palpable, erythematous lesions on the trunk. Biopsy of a rose spot reveals nests of mononuclear cells and usually yields S typhi. However, rose spots are often sparse, transient, and difficult to spot on dark-skinned children. The natural course of illness is persistence of fever for 2 to 3 weeks, with slow recovery.
Complications are common.30,31 Most fatalities are the result of intestinal hemorrhage or perforation, resulting from necrosis of infected Peyer patches.32 The overall mortality is 3% to 6% with treatment. Predictors of mortality include intestinal perforation, seizures, septic shock, pneumonia, delirium, and coma.26,31,33 Late focal infections, such as meningitis, endocarditis, osteomyelitis, and pneumonia, are rare. Relapse, a recurrence of the manifestations of typhoid fever after initial clinical response, occurred in 8% to 12% of patients who did not receive antimicrobial therapy, but may be higher in the antibiotic era.
Children younger than 2 years of age often have mild illness, often resembling a mild, nonspecific febrile illness. Classic typhoid fever can occur in this age group, although it is the exception.28,34
 DIAGNOSIS
DIAGNOSIS
Salmonella should always be considered in a child with gastroenteritis. More severe disease, fever, headache, evidence of dysentery, immune deficiency, recent immigration from endemic areas, exposure to reptiles, under-cooked meat or eggs, or an ongoing common source outbreak should increase suspicion.
The diagnosis is made by stool culture. If fresh stool cannot be obtained, a rectal swab can be cultured. The yield is higher from fresh stool, and the use of enrichment broths in the microbiology lab also improves sensitivity. Blood cultures are negative in the majority of children with Salmonella gastroenteritis, and agglutination tests are of no value. Gastroenteritis with fever, especially in a child under 2 years old, is usually an indication for obtaining a blood culture. Cerebrospinal fluid cultures should be obtained when salmonellosis is suspected in infants younger than 3 months of age, even in the absence of elevated temperature, because of the increased risk in this age group.
For suspected enteric fever, serial blood cultures should be obtained. In untreated patients with typhoid fever, 3 blood cultures during the first week have approximately a 90% yield. Bone marrow culture is the most sensitive procedure for recovery of S typhi. Culture of bile obtained by a swallowed capsule (string test) is also sensitive, but not as sensitive as the combination of blood and bone marrow cultures.
The Widal test is problematic. It measures the titer of agglutinating serum antibodies against the O and H antigens of S typhi. In untreated disease, only one half to two thirds of patients have a fourfold or greater increase in titer. Moreover, the Widal test is not specific. In the future, using polymerase chain reaction techniques may increase the sensitivity and turnaround time for detection of bacteremia.
 TREATMENT
TREATMENT
Uncomplicated Salmonella gastroenteritis requires no antimicrobial therapy; antibiotics do not shorten the clinical illness, as demonstrated in carefully conducted trials. In addition, antimicrobials may select for resistant strains and prolong Salmonella carriage. Antimicrobial therapy should be given to patients with enteric fever, bacteremia from nontyphoid strains, and disseminated infection with localized suppuration. Antimicrobial therapy also should be considered in infants younger than 3 to 6 months and in patients with enterocolitis who have HIV disease or other underlying conditions that impair host resistance.
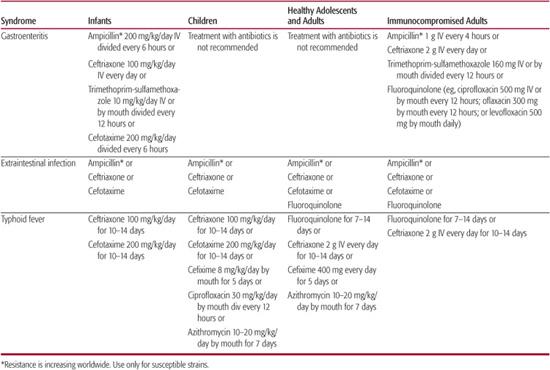
Ampicillin, chloramphenicol, and trimethoprim-sulfasoxazole have in vitro activity and historically have been successful in treating patients with nontyphoid Salmonella infections (Table 283-1). Resistance to these agents has increased in recent years. A highly drug-resistant clone, S typhimurium DT (definitive type) 104, has spread explosively in the United States and Europe. Therefore, all isolates should be tested for susceptibility. Cefotaxime is useful for ampicillin-resistant strains and is the drug of choice for Salmonella meningitis. Therapy for uncomplicated bacteremia is usually given for 10 to 14 days; at least 7 days of therapy should be intravenous. Meningitis should be treated for at least 3 weeks.
Ampicillin, amoxicillin, and trimethoprimsulfasoxazole are effective therapy for typhoid fever caused by susceptible S typhi. Multidrug-resistant S typhi is a rapidly emerging problem worldwide. Ampicillin, chloramphenicol, and trimethoprim-sulfasoxazole may no longer be reasonable choices for empiric therapy before susceptibility tests are available, and fluoroquinolones can be considered.31 Short courses of oral cefixime demonstrate acceptable success rates, but the time to clinical improvement is slower than with ceftriaxone or fluoroquinolones. Antimicrobial agents that have been used successfully in the treatment of resistant S typhi strains are cefotaxime, ceftriaxone, cefixime, aztreonam, ofloxacin, and ciprofloxacin, but isolates resistant to each of these classes have been reported. Patients whose isolates have elevated minimum inhibitory concentrations to ciprofloxacin or resistance to nalidixic acid have higher failure rates when treated with fluoroquinolones.35 Azithromycin has been used for fluoroquinolone-resistant typhoid, but the optimal treatment is unknown.36,37 These agents might be predicted to work for bacteremia with resistant nontyphoid Salmonella as well.
Survival of patients with delirium, stupor, or coma associated with typhoid fever is improved by brief, high-dose corticosteroid therapy administered concurrently with antibiotics.31 Dexamethasone has been used at an initial dose of 3 mg/kg followed by 8 doses of 1 mg/kg every 6 hours. Aggressive surgical intervention, together with broad-spectrum antibiotics (including anti-Salmonella therapy), has improved survival in typhoid fever complicated by intestinal perforation with peritonitis.
The chronic asymptomatic carriage of S typhi can be extremely difficult to eradicate, especially if there is obstructive hepatobiliary disease such as gallstones. Success has been achieved with a combination of 6 weeks of ampicillin or amoxicillin with probenecid in patients who have normally functioning gall-bladders without evidence of cholelithiasis. In adults, ciprofloxacin has been somewhat successful in eradicating the organism in chronic carriers. Cholecystectomy is recommended for carriers who have relapsed after therapy or who cannot tolerate antimicrobial therapy. Patients who excrete nontyphoid Salmonella usually do not need antimicrobial therapy.
The mortality of Salmonella infections ranges from 0.5% to 1.4%. Most deaths are associated with bacteremia, sepsis, or meningitis. Malnutrition, extremes of age, and underlying disease strongly influence mortality. The fatality rate for typhoid fever is less than 2% for industrialized nations, but approaches 35% for developing countries.
 PREVENTION
PREVENTION
Improvements in sanitation, waste disposal, and safe drinking water led to a dramatic decrease in S typhi infections, but have had little impact on the control of nontyphoid Salmonella. Prevention of nontyphoid Salmonella involves many fronts. The amount of Salmonella, particularly antimicrobial-resistant Salmonella, reaching the consumer depends on practices in agriculture.
Three vaccines against typhoid fever are available for civilian use in the United States: a parenteral heat-phenol-inactivated vaccine; an orally administered, live-attenuated oral vaccine prepared from the Ty21a strain of S typhi; and an injectable vaccine made from purified Vi polysaccharide.38,39 The vaccines are of roughly equal efficacy. However, the oral vaccine and the Vi polysaccharide cause significantly fewer side effects and are preferred. The oral Ty21a vaccine is licensed for children 6 years and older, although it is immunogenic in children 2 years and older. It requires 4 oral doses and must be repeated every 5 years. The Vi-polysaccharide vaccine is licensed for children older than 2 years. It requires a single injection, but must be repeated every 2 years. 
SHIGELLA
Shigella are divided into 4 groups and over 40 serotypes based upon serologic and biochemical reactions: S dysenteriae (group A), S flexneri (group B), S boydii (group C), and S sonnei (group D). Shigella are gram-negative, nonlactose-fermenting aerobic, nonmotile bacilli, closely related to E coli. They do not survive well in the environment, and delays in plating stool specimens may significantly reduce the recovery rate.  Even optimal handling of stool specimens may not result in isolation of the organism.
Even optimal handling of stool specimens may not result in isolation of the organism.
Table 283-1. Antimicrobial Therapy for Patients with Salmonella Infections
 EPIDEMIOLOGY AND PATHOPHYSIOLOGY
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
The epidemiology of shigellosis differs between developed and less-developed countries. S sonnei is the most frequently isolated serotype in the United States and western Europe, accounting for 60% to 80% of Shigella infections; S flexneri is second in frequency. S dysenteriae serotype 1 (the Shiga bacillus) is rare in developed countries, but a major problem in sub-Saharan Africa and the Indian subcontinent.
High attack rates occur among children in childcare centers, persons in nursing homes, residents of facilities for the mentally ill, and persons living on Native American reservations. The highest attack rate for shigellosis is in children 1 to 4 years old (eFig. 283.2  ). Breast-feeding is clearly protective.
). Breast-feeding is clearly protective.
Shigella infection is acquired through fecaloral exposure. Unlike Salmonella infections, however, person-to-person transmission plays a key role. In volunteer studies, as few as 10 to 100 organisms can cause disease.
Humans are the primary reservoir of Shigella; higher primates can become infected but do not play a significant role in the epidemiology. Crowding, poor sanitation, inadequate supplies of water for washing, lack of soap, and the presence of diapered children are risk factors for the spread of Shigella. Outbreaks of shigellosis in childcare centers are common. Outbreaks have also occurred as a consequence of swimming in contaminated lakes and pools. There have been many foodborne outbreaks from an ever-expanding list of vehicles; fresh fruits and vegetables have assumed a prominent role in recent years. Shigella infections show a distinct seasonal peak during July through October, but infections occur year-round.40
The role of asymptomatic carriers in the epidemiology of shigellosis is not completely understood. In one study, 17% of children excreted Shigella for at least 1 month after the acute illness, and 11% excreted Shigella for at least 2 months. In highly exposed populations, asymptomatic carriage and/or prolonged excretion may be more common.
The cardinal features of the pathogenesis of shigellosis are its ability to invade cells and to incite an inflammatory response.41-44 In the colon, Shigella bind to M cells and translocate across them. Shigella then invade enterocytes, lyse the cytoplasmic vacuoles, and move to the cytoplasm, where they divide. The pathologic changes that accompany this include superficial ulcerations of the mucosa, inflammation, hemorrhages, edema, and friability. Involvement is typically worse in the rectosigmoid and distal colon.
S dysenteriae type 1 also encodes genes for Shiga toxin, a potent inhibitor of protein synthesis. Shiga toxin is responsible for the increased virulence of this organism and its association with the hemolytic uremic syndrome.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The clinical manifestations of shigellosis vary. The incubation period can be as short as 12 hours or as long as 5 days, but most often is 24 to 48 hours. Most illness begins with fever, malaise, anorexia, and occasionally vomiting or headache. Diarrhea usually begins as watery diarrhea and may progress within hours or days to dysentery. Typical symptoms of dysentery include frequent small-volume stools containing mucus and blood associated with lower abdominal cramps and tenesmus. However, the diarrhea may remain watery and copious. Asymptomatic infection also occurs.
Physical findings include fever, systemic toxicity, increased bowel sounds, and lower abdominal tenderness. The child may have signs of dehydration. Rectal prolapse may occur in 5% to 8% of patients. In general, S sonnei causes milder illness with fewer complications, S flexneri tends to be more severe, and S dysenteriae tends to cause the most severe dysentery and extraintestinal complications. The white blood cell count is usually elevated. Leukemoid reactions with as many as 50,000 cells/μL occasionally occur.
Shigellosis is less common in neonates and young infants than in children older than 1 year (eFig. 283.2  ), but infants and neonates are more likely to be severely dehydrated or hypothermic, are twice as likely to die, and are less likely to have classic findings such as high fever, bloody diarrhea, abdominal tenderness, and rectal prolapse.45 Thus, a high index of suspicion and a low threshold for obtaining stool cultures are appropriate for young infants with diarrhea.
), but infants and neonates are more likely to be severely dehydrated or hypothermic, are twice as likely to die, and are less likely to have classic findings such as high fever, bloody diarrhea, abdominal tenderness, and rectal prolapse.45 Thus, a high index of suspicion and a low threshold for obtaining stool cultures are appropriate for young infants with diarrhea.
A variety of complications of shigellosis occur.43,46,47 Dehydration, hypoglycemia, hyponatremia, hypernatremia, and hypokalemia can occur; hypoglycemia and hyponatremia are associated with an increased mortality rate. Seizures are a common extraintestinal manifestation.48 Isolated seizures are usually not associated with any long-term neurologic sequelae. Ekiri, a severe toxic encephalopathy initially described in Japan, manifests as severe dysentery, sensory disturbances, convulsions, and rapid progression to death.
Table 283-2. Antimicrobial Therapy for Patients with Shigellosis
Bacteremia is uncommon in healthy children, but is more common with HIV infection and severe malnutrition. Extraintestinal suppurative complications such as osteomyelitis, meningitis, septic arthritis, and splenic abscess are rare. The hemolytic uremic syndrome is an important complication of S dysenteriae type 1 infection, reported in 1% to 4% of children (see Chapter 472); it is seen occasionally with S flexneri.49,50 Reactive arthritis or Reiter syndrome (arthritis, urethritis, iritis in conjunction with HLA-B27) are unusual postinfectious complications of Shigella, usually in adolescents or adults.51
 DIAGNOSIS
DIAGNOSIS
Shigella should be suspected in children with fever and diarrhea, particularly if there are seizures, or in those children with small-volume diarrhea with abdominal cramping, blood, white blood cells, or mucus in the stool. Many patients with S sonnei, however, do not have bloody diarrhea. The differential diagnosis of bloody diarrhea is discussed in Chapter 387.
The diagnosis hinges on the recovery of Shigella from a fresh stool specimen or a rectal swab. Recovery is easier early in the course of the disease. Shigella may not survive transportation. If rectal swabs are used, they should be placed in appropriate transport media, such as Cary-Blair media. Specimens should be processed immediately by the clinical microbiology laboratory. Even with optimal handling, false-negative cultures will occur in about 20% of cases.
Presumptive identification of Shigella requires at least 48 hours; definitive identification may require 72 hours. Polymerase chain reaction has been used to detect Shigella in stool and appears to be very sensitive, but it is not commercially available. In developed countries, the frequency of Shigella and E coli O157:H7 infection in children with bloody diarrhea is similar. Making the distinction is critical, because shigellosis responds to antimicrobial therapy but E coli O157:H7 infections do not, and some evidence suggests that antimicrobials increase the risk of hemolytic uremic syndrome. Fortunately, new enzyme-based immunoassays for Shiga toxin allow the diagnosis of E coli O157:H7 and other enterohemorrhagic E coli in about 24 hours.
 TREATMENT
TREATMENT
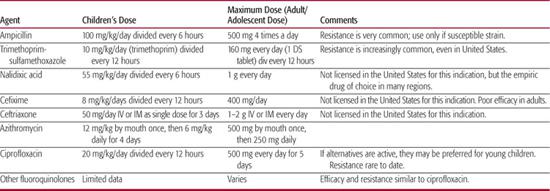
Fluid and electrolyte therapy are key components of management. Although shigellosis is a self-limited disease for most patients, antibiotic treatment during acute dysentery will reduce the duration of fever and diarrhea. Table 283-2 lists antimicrobial therapy for patients with shigellosis. Treatment of milder disease or later in the course has only modest clinical benefit but leads to more rapid cessation of shedding, usually within 1 to 2 days.
Antibiotic-resistant Shigella have emerged rapidly throughout the world.43,52-54 Strains of S dysenteriae type 1 resistant to virtually all antibiotics except fluoroquinolones have spread widely in sub-Saharan Africa, the Indian subcontinent, and Latin America. Unfortunately, strains resistant to fluoroquinolones are now being reported. Ampicillin was once the drug of choice for Shigella, but resistance is now close to universal. Trimethoprim-sulfamethoxazole is effective for sensitive strains. However, resistance is extremely common in Southeast and South Asia and Latin America and has increased rapidly in the United States. Trimethoprim-sulfamethoxazole therefore can no longer be considered a reliable drug for empiric therapy without considering local epidemiology.
Resistant strains of S sonnei and S flexneri are often susceptible to fluoroquinolones, nalidixic acid, ceftriaxone, cefixime, and azithromycin. Nalidixic acid is inexpensive and effective in children. Azithromycin was effective in a single trial, but the widespread use of this drug may lead to more resistance.55 For severe shigellosis, IV or IM ceftriaxone is effective.56 Although fluoroquinolones are not approved for use in children because of lingering concerns over arthropathy, 2 clinical trials in children with severe shigellosis suggested that ciprofloxacin or norfloxacin are safe and effective.57-59
Antidiarrheal agents that reduce gastrointestinal tract motility should not be used in infants and children with infectious diarrhea. Their use in children with shigellosis is associated with toxic megacolon and with hemolytic uremic syndrome in patients with E coli O157:H7.
Shigellosis usually resolves completely in 7 to 10 days if untreated. A postdiarrheal enteropathy may occur, particularly following S dysenteriae infection. Growth delay and exacerbation of malnutrition may follow shigellosis, particularly in children with preexisting malnutrition. The mortality rate depends on the setting and on the health of the child. In developed countries the mortality is less than 1%. In developing countries, the mortality can range from 10% to 30%. Risk factors for death include infection with S dysenteriae type 1, malnutrition, very young age, and bacteremia.
 PREVENTION
PREVENTION
Simple measures of hygiene can greatly reduce the incidence of Shigella infections in resource-poor settings. These measures include the provision of appropriate sanitation, safe drinking water, and soap and water for hand washing. Control of flies reduces disease. Provision of narrow-mouthed water containers that cannot be contaminated by dirty hands is a cost-effective prevention tool. Breast-feeding is a practical strategy to prevent disease in infants.
Prevention of shigellosis in developed countries depends on control of person-to-person transmission and identification of food-borne outbreaks. Within childcare centers control measures include adequate number of sinks, methods to clean diaper-changing surfaces, prohibiting food handlers from diaper changing, cohorting of sick children, and frequent staff education. In other institutional outbreaks, cohorting and contact isolation are important measures. Antibiotic treatment of infected persons may curtail outbreaks in closed settings. Antibiotic prophylaxis is ineffective and risks the emergence of resistance.
Several candidate vaccines are being tested, including polysaccharide conjugate vaccines and molecular constructs in live vectors.44
Table 283-3. Characteristics of Escherichia coli Associated with Diarrhea
DIARRHEA-CAUSING ESCHERICHIA COLI
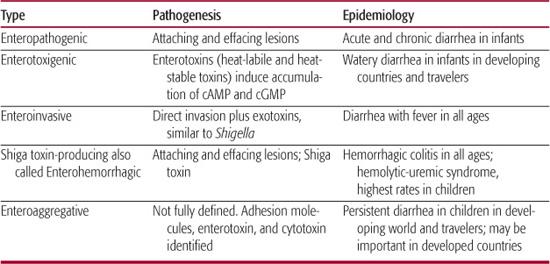
Escherichia coli are gram-negative, lactose-fermenting, motile, facultative bacilli belonging to the family Enterobacteriaceae. E coli can be grouped by serotype, defined by the 171 somatic (O) and 56 flagellar antigens. Thus, E coli O157:H7 is an example of a specific serotype. E coli are the most common flora of the gastrointestinal tract and probably serve useful symbiotic functions. Most are nonpathogenic, but some possess specific virulence traits that enable them to cause meningitis, urinary tract infection, or diarrhea.60,61 The diarrhea-causing E coli fall into 5 distinct phenotypes (Table 283-3). Each phenotype possesses unique genes encoding virulence traits, each with its own pathogenesis.
Because E coli is the most common facultative organism in stool, identifying diarrhea-causing E coli in stool specimens is difficult. 
Enterohemorrhagic E coli are able to cause an attaching and effacing lesion in intestinal mucosa and secrete Shiga toxin  .62 Because Shiga toxin is the defining virulence factor, the preferred terminology for this group of organisms is now Shiga toxin-producing E coli. At least 12 serotypes of E coli are Shiga toxin-producing E coli, but 1 serotype, E coli O157:H7 is the predominant strain in much of the world.
.62 Because Shiga toxin is the defining virulence factor, the preferred terminology for this group of organisms is now Shiga toxin-producing E coli. At least 12 serotypes of E coli are Shiga toxin-producing E coli, but 1 serotype, E coli O157:H7 is the predominant strain in much of the world. 
Enterotoxigenic E coli belong to many serotypes. They produce the enterotoxins heat-stable toxin and heat-labile toxin, which cause secretory diarrhea without invading or damaging enterocytes. They can be identified in the laboratory by detection of enterotoxin by using enzyme immunoassay or by a bioassay  .
.
Enteropathic E coli
Enteropathic E coli are an important cause of diarrhea in infants in developing countries and were responsible for numerous nursery outbreaks in industrialized countries during the 1950s and 1960s. Traditionally defined by serotype, they are now defined by localized adherence pattern to Hep2 cells and by virulence genes. 
Enteroinvasive E coli
Enteroinvasive E coli are strains of E coli that closely resemble Shigella in their genetics, biochemical characteristics, and clinical manifestations. Identification of enteroinvasive E coli was traditionally performed by isolating strains of E coli with a positive Sereny test (guinea pig keratoconjunctivitis). Gene probes for the invasiveness genes ipaC are now used to screen colony blots of E coli for enteroinvasive E coli. Polymerase chain reaction has been used experimentally.
Enteroaggregative E coli
Enteroaggregative E coli are the most recent phenotype of E coli demonstrated to cause diarrhea. They are defined as strains that do not secrete heat-labile toxin or heat-stable toxin and that adhere to Hep-2 cells or a coverslip with a “stacked brick” pattern of adherence.67
 EPIDEMIOLOGY
EPIDEMIOLOGY
All diarrhea-causing E coli are transmitted by fecal-oral contact, but they differ in the infectious dose; the susceptible population; the geographic patterns; and the relative importance of food, water, and person-to-person transmission.
E coli O157:H7 and other Shiga toxin-producing E coli have emerged as some of the most important enteric pathogens in developed countries. They are a relatively common cause of nonbloody and bloody diarrhea in developed countries of North and South America, Europe, and Japan.62,69-71 In a multicenter study in the United States, E coli O157:H7 were the third or fourth most commonly identified stool pathogen. Shiga toxin-producing E coli are responsible for upward of 90% of postdiarrheal hemolytic uremic syndrome cases and an unknown proportion of cases of thrombotic thrombocytopenic purpura. E coli O157:H7 is the predominant strain in most regions.  The incidence is highest among children younger than 5 years (10.3/100,000 person years) but infection occurs in all age groups.72 Rates of infection are higher in northern states, suggesting complex local epidemiology.4E coli O157:H7 infections show a clear seasonal pattern, with peak incidence in the summer and fall in the Northern Hemisphere.
The incidence is highest among children younger than 5 years (10.3/100,000 person years) but infection occurs in all age groups.72 Rates of infection are higher in northern states, suggesting complex local epidemiology.4E coli O157:H7 infections show a clear seasonal pattern, with peak incidence in the summer and fall in the Northern Hemisphere.
The natural reservoir of Shiga toxin-producing E coli is dairy cattle; however, other animals that carry the organism include goats, sheep, pigs, deer, and elk. Outbreaks are most often the result of consumption of hamburger meat or raw milk. Several outbreaks have followed swimming in contaminated water in lakes, ponds, and swimming pools. Other outbreaks have been the result of consumption of roast beef, apple cider, unpasteurized apple juice, salami, municipal water, and produce, including leaf lettuce, spinach, alfalfa, and radish sprouts. Most cases are sporadic, and consumption of undercooked hamburger and farm visits are risk factors for sporadic cases.73,74 The infectious dose for E coli O157:H7 is low, estimated from 1 outbreak to be less than 700 organisms. Person-to-person transmission has been documented in institutions, childcare centers, homes with young children, and, rarely, in hospitals.62,75
Enterotoxigenic E coli is an important cause of diarrhea in 2 groups: young children in developing countries and travelers to developing countries. Twenty percent to 40% of travelers’ diarrhea is due to enterotoxigenic E coli. Transmission generally results from contamination of water and food in conditions of poor sanitation and requires a relatively high inoculum. Enterotoxigenic E coli outbreaks were once thought to be extremely rare in developed countries. However, in recent years, several outbreaks have been identified in the United States.76,77
Enteroaggregative E coli was initially identified as a pathogen in developing countries and later associated with persistent diarrhea among persons with HIV infection.78 Recent studies showing that these organisms were an important cause of diarrhea in children in Baltimore and New Haven in the United States have challenged that assumption.79
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Shiga Toxin-Producing E coli
Enterohemorrhagic E coli are able to cause an attaching and effacing lesion in intestinal mucosa and secrete Shiga toxin.62 Because Shiga toxin is the defining virulence factor, the preferred terminology for this group of organisms is now Shiga toxin-producing E coli. At least 12 serotypes of E coli are Shiga toxin-producing E coli, but 1 serotype, E coli O157:H7 is the predominant strain in much of the world. The incubation period for Shiga toxin-producing E coli is usually 3 to 4 days, although it ranges from 1 to 9 days. E coli O157:H7 are quite acid tolerant and probably are less affected by the gastric acid barrier than are other enteric pathogens. On reaching the intestine, Shiga toxin-producing E coli adhere to enterocytes, primarily in the colon. A characteristic attaching and effacing lesion occurs, similar to that which occurs with enteropathic E coli. Histology shows edema and submucosal hemorrhage. The defining virulence factor for Shiga toxin-producing E coli is the ability to produce Shiga toxin (Stx), one of the most potent protein inhibitors of protein synthesis known.62,70,71
Enterotoxigenic E coli
Enterotoxigenic E coli belong to many serotypes. They produce the enterotoxins heat-stable toxin and heat-labile toxin, which cause secretory diarrhea without invading or damaging enterocytes. The pathogenesis of enterotoxigenic E coli is fairly similar to that of Vibrio cholerae. Symptoms follow an incubation period of 14 to 48 hours. Enterotoxigenic E coli colonize the surface of the small-bowel epithelium but do not invade. Binding is mediated by surface fimbriae (or pili), referred to as colonization fimbriae.
Enteropathic E coli
Enteropathic E coli are an important cause of diarrhea in infants in developing countries and were responsible for numerous nursery outbreaks in industrialized countries during the 1950s and 1960s. Traditionally defined by serotype, they are now defined by localized adherence pattern to Hep2 cells and by virulence genes. The incubation period for enteropathic E coli is 6 to 48 hours. Enteropathic E coli adhere to the epithelial cell through a process thought to be mediated by the bundle-forming pilus encoded in the E coli adherence factor (EAF) plasmid.
Enteroinvasive E coli
Enteroinvasive E coli are strains of E coli that closely resemble Shigella in their genetics, biochemical characteristics, and clinical manifestations. The pathophysiology of enteroinvasive E coli is virtually identical to Shigella. It invades and divides within enterocytes, causing intense inflammation.
Enteroaggregative E coli
Enteroaggregative E coli are the most recent phenotype of E coli demonstrated to cause diarrhea. They are defined as strains that do not secrete heat-labile toxin or heat-stable toxin and that adhere to Hep-2 cells or a coverslip with a “tacked brick” pattern of adherence.67 This test is only available in research laboratories, is tedious, and requires care and expertise. Several genetic markers of virulence factors have been found that identify enteroaggregative E coli, including EAST1, CVD432, and several adherence markers that can be detected by probe or polymerase chain reaction. The optimal use of these markers remains unclear.67,68 Much less is understood about the pathogenesis of diarrhea caused by enteroaggregative E coli. Not all strains cause diarrhea in laboratory animals or in human volunteers. Enteroaggregative E coli adhere to small-bowel mucosa.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Shiga toxin-producing E coli most often cause hemorrhagic colitis, characterized by vomiting, bloody diarrhea, and severe abdominal cramps. Fecal leukocytes may be present but are not prominent. Asymptomatic carriage and non-bloody diarrhea also occur. Hemolytic-uremic syndrome—the triad of thrombocytopenia, hemolytic anemia, and renal failure—occurs in 5% to 10% of children infected with E coli O157:H7 (see Chapter 472), although this varies widely among outbreaks. The rate may be lower for non-O157 strains. Leukocytosis during the diarrheal illness has been consistently reported to be a predictor of hemolytic-uremic syndrome. Risk factors for hemolytic-uremic syndrome include young age, use of antimotility agents, and possibly, use of antimicrobial agents. The data implicating antimicrobial agents are based on in vitro studies that demonstrate that antimicrobial agents upregulate toxin production82-84 and epidemiologic studies. A limited number of retrospective epidemiologic studies85 and a single prospective study86 demonstrated an association between antibiotic use and hemolytic-uremic syndrome or death. In the absence of a well-designed prospective study, many experts continue to recommend avoiding antibiotics during Shiga toxin-producing E coli infection.87 In adults, Shiga toxin-producing E coli infection may lead to thrombotic thrombocytopenic purpura.
Enterotoxigenic E coli causes large-volume watery diarrhea. Fever and vomiting may occur, but are uncommon. The diarrhea may be mild and self-limited, or it may lead to massive fluid loss and dehydration. Blood, mucus, or fecal white cells are very uncommon. Duration is typically less than 1 week.
Enteropathic E coli diarrhea typically occurs in children less than 2 years old. Diarrhea is usually watery and profuse. Vomiting and low-grade fever are common. While most cases are associated with acute disease, persistent diarrhea may occur.
Enteroinvasive E coli causes abdominal cramps, tenesmus, fever, and small-volume stools. Blood and mucus may be present, but may be less common than with some of the more virulent strains of Shigella.
Enteroaggregative E coli most often occurs as a watery diarrhea without vomiting and with low-grade fever. Mucoid diarrhea is common, and in some series bloody diarrhea occurs in up to one third of patients. Of particular importance is the association of enteroaggregative E coli with persistent diarrhea. In malnourished children, persistent diarrhea is associated with malnutrition and contributes to increased mortality.
 DIAGNOSIS
DIAGNOSIS
Diagnosis of infection by diarrhea-causing E coli is difficult. Techniques for the diagnosis of Shiga toxin-producing E coli are widely available in clinical laboratories, although many do not test routinely. Diagnosis should be vigorously pursued in outbreaks and in the evaluation of patients with persistent diarrhea, especially in the setting of foreign travel.
E coli O157:H7 can be identified by screening with sorbitol MacConkey agar, an inexpensive technique that should be available in all clinical microbiology laboratories. A few commercial reference laboratories offer the Hep-2 adherence assay for diagnosing enteropathic E coli and enteroaggregative E coli. Multiplex polymerase chin reaction offers promise for detecting several phenotypes at once.65,88
Enterotoxigenic E coli cannot be identified by biochemical characteristics or serotype. Definitive diagnosis is based on detection of toxin in E coli colonies in the suckling mouse assay, the Y1 adrenal cell assay, enzyme immunoassay, DNA probes, or polymerase chain reaction primers directed against the genes encoding heat-labile toxin and heat-stable toxin.
Serotyping was once the gold standard for identifying enteropathic E coli strains; it is no longer considered the primary method. According to a consensus definition, enteropathic E coli strains are those that can cause an attaching and effacing lesion in the Hep-2 adherence assay and that lack Stx. Genotypic diagnosis relies on DNA probes and polymerase chain reaction primers for the eae gene, the bfp gene, and the EAF plasmid.
Enteroinvasive E coli can be diagnosed by demonstrating that an isolate with the biochemical characteristics of E coli contains Shigella invasiveness genes. An enzyme immunoassay to detect the ipaC gene has been developed that can detect enteroinvasive E coli and Shigella. It is not yet widely available.
The gold standard for diagnosing enteroaggregative E coli is the Hep-2 adherence assay, in which tested E coli isolates demonstrate the stacked brick configuration. The utility of the EAST1 and CVD432 probes appear to vary by region.
 TREATMENT
TREATMENT
As for all diarrheal illness in children, the cornerstone of therapy is careful replacement of fluids and electrolytes (Chapter 385).87 The treatment of Shiga toxin-producing E coli is supportive. As noted earlier, the use of antimicrobial agents in Shiga toxin-producing E coli may be associated with an increased risk of hemolytic-uremic syndrome. Although this remains controversial, there are no data supporting any clinical benefit, so it is prudent to avoid all antimicrobials.
Antimicrobial therapy is clearly useful in shortening the course of enterotoxigenic E coli infections in travelers, as demonstrated in numerous studies in many settings. Trimethoprimsulfamethoxazole, doxycycline, bicampicillin, furazolidine, rifaximin, and fluoroquinolones have demonstrated efficacy. However, resistance to trimethoprim-sulfamethoxazole is common in some regions. Treatment of enteropathic E coli with trimethoprim-sulfamethoxazole, oral gentamicin, and oral colistin are effective, although symptomatic therapy may suffice. Antibiotics used to treat Shigella can be used in enteroinvasive E coli, although clear data are lacking. The optimal treatment of enteroaggregative E coli infection is unknown, but small studies have reported clinical response to ciprofloxacin among HIV-infected patients and travelers with enteroaggregative E coli infection.89,90 Rifaximin was more effective than placebo among travelers in a small study.89
Antimotility agents should be avoided in persons with inflammatory or bloody diarrhea and in infants and children with diarrhea. Antimotility agents in low dose, and for a brief period, are safe for treating secretory diarrhea in adults. Bismuth subsalicylate reduces the severity of diarrhea in patients with enterotoxigenic E coli infections, although salicylate absorption occurs. The general rule is that the amount of salicylate absorbed from 30 mL of Pepto-Bismol is similar to that of one 325-mg aspirin tablet.
Currently, Shiga toxin-producing E coli are associated with the greatest morbidity in developed countries. Five percent to 10% of infected patients develop hemolytic-uremic syndrome, for which the mortality rate is 2% to 5%. The major complications of ETEC infection are related to dehydration and to electrolyte disturbances. However, enterotoxigenic E coli contributes a substantial proportion of the approximately 3 million deaths that occur as a consequence of diarrhea among infants and young children in developing countries. Although several nursery epidemics caused by enteropathic E coli in the 1940s and 1950s reported mortality rates approaching 50%, the current mortality is less than 1% in developed countries. The persistent diarrhea associated with enteroaggregative E coli is associated with growth failure and increased mortality. As rehydration services improve in developing countries, persistent diarrhea accounts for an increasing proportion of diarrheal mortality.
 PREVENTION
PREVENTION
The most important measures for prevention of enterotoxigenic E coli, enteropathic E coli, enteroaggregative E coli, and enteroinvasive E coli are improvements in sanitation, including proper disposal of human waste, access to water and soap for hand washing, and clean sources of food for weaning. Breast-feeding is protective and should be encouraged. Nosocomial transmission of diarrhea causing E coli occurs; therefore, careful enteric precautions should be followed.
To prevent Shiga toxin-producing E coli, hamburger and other beef products should be cooked thoroughly and protected from contamination from raw meat. Unpasteurized milk and apple juice should be avoided. Because several outbreaks of E coli O157:H7 were caused by diapered children in swimming pools and ponds, this is a potential area for intervention.
To prevent enterotoxigenic E coli infection, travelers to developing countries should avoid ice, salads, and those raw fruits and vegetables without peels. They should drink only boiled or carbonated water or beverages or bottled water from a reliable source. Infant formula should be prepared from boiled water. Routine use of prophylactic antibiotics is not recommended, particularly for children. Bismuth subsalicylate can be used for prophylaxis in adolescents and adults, but the aspirin exposure represents a relative contraindication for young children.
Because preliminary studies with inactivated enterotoxigenic E coli vaccines prove that protective vaccines are feasible, several approaches are under active investigation.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


