Fig. 20.1
Threshold of hypoxaemia at different altitudes
Changing the point at which hypoxaemia is defined and oxygen is given results in a major variation in the amount of oxygen used. A report from one hospital found that 13 % of children with pneumonia were hypoxaemic using a definition of SpO2 <85 %, 26 % were hypoxaemic using SpO2 <90 and 44 % were hypoxaemic using SpO2 <93 % (Laman et al. 2005).
The best cut-off point for giving supplemental oxygen may be the level of blood oxygen that is associated with increased morbidity or risk of death or delayed recovery, rather than a certain level of haemoglobin–oxygen saturation below normal for the population. With normal cardiac output, haemoglobin concentration and pH, arterial oxygen saturations of 68 % or more are probably not dangerous (Nunn 1993). However, there are few data about the exact SpO2 below which the risk of adverse outcomes increases. This risk will be different for different ages, disease states and comorbidities and at different altitudes, and a safe margin for error is required.
The gold-standard measure for the oxygen content of the blood is the arterial oxygen tension or PaO2 (measured in mm Hg or kilopascals). PaO2, however, can only be measured by blood gas analysis. This method is invasive, painful and distressing to the patient, and blood gas machines and reagents are very expensive and, therefore, not appropriate in most district hospitals in developing countries. Therefore, we use SpO2, which is related to PaO2, to define hypoxaemia in these guidelines (see Fig. 20.2).
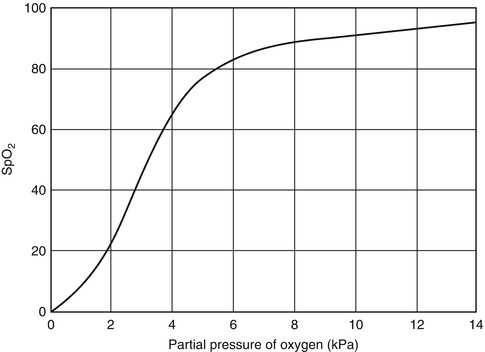

Fig. 20.2
Haemoglobin–oxygen dissociation curve
In practice, most studies have adopted a threshold at which to give oxygen of SpO2 <90 %. This corresponds to the beginning of the steep part of the haemoglobin–oxygen dissociation curve, which is shown in Fig. 20.2. Small reductions in SpO2 below 90 % may represent dangerous falls in PaO2. This represents a safe margin for error where oxygen supplies are sufficient.
There will be conditions that require oxygen therapy at higher thresholds than 90 % SpO2. These are conditions where oxygen delivery from the lungs to body tissues is seriously impaired or where vital organs may be particularly susceptible to low oxygen levels. Examples include severe anaemia, severe heart failure, severe sepsis or brain injury. In these conditions, many clinicians recommend giving oxygen if the SpO2 is <94 %.
It is important to note that small changes in SpO2 between 90 and 100 % reflect large changes in PaO2, because the haemoglobin–oxygen dissociation curve is relatively flat. Below an SpO2 of 90 %, however, the curve is steep and small falls in PaO2 may result in much larger falls in SpO2.
The clinical signs of hypoxaemia have been evaluated in many studies and reviewed (Rojas et al. 2009; Ayieko and English 2006). In situations where the oxygen supply is very limited, for children aged over 2 months, provide oxygen according to the priority listing suggested in Table 20.1. Infants aged <2 months with signs of severe respiratory distress (tachypnoea, severe chest indrawing, head nodding or grunting) should be given oxygen because hypoxaemia puts them at greater risk of apnoea and death.
Table 20.1
Clinical indications for oxygen therapy
Clinical presentation for severe pneumonia with | Priority for oxygen |
|---|---|
Central cyanosis | Very high priority |
Decreased consciousness, unresponsiveness or responsive to painful stimuli only | Very high priority |
Head nodding or grunting | Very high priority |
Severe palmar or conjunctival pallor (severe anaemia) with severe lower chest wall indrawing or fast breathing | Very high priority; high priority should also be given to urgent correction of the underlying abnormality (i.e. blood transfusion and/or antimalarials) |
Acute coma or convulsions lasting more than 15 min | Very high priority until respiratory effort has returned to normal; also protect airway and ensure adequate ventilation |
Inability to drink or feed | High priority |
Severe chest indrawing | Priority |
Even the best combinations of clinical signs commonly misdiagnose hypoxaemia in some patients with normal oxygen saturation or fail to detect some hypoxaemic patients. Pulse oximetry has been found to correctly identify 20–30 % more children who have hypoxaemia than will be found using clinical signs alone (Usen et al. 1999; Weber et al. 1997; Duke et al. 2002a). When used correctly, pulse oximetry provides reliable monitoring with little or no distress to the patient; in industrialized countries, it is the accepted standard for detecting hypoxaemia (Schnapp 1990).
When monitoring with oximetry, as a general rule, any child with an SpO2 <90 % should receive oxygen. This rule best applies to health facilities located between sea level and 2,500 m above sea level and where oxygen supplies are ample (such as when using concentrators) for altitudes higher than 2,500 m.
Where there is sufficient oxygen to treat all children with hypoxaemia, it is the practice in some hospitals to give oxygen if the SpO2 is <93 %. Some doctors suggest oxygen should be “discretionary” between an SpO2 of 90 and 92 % and “mandatory” at SpO2 <90 %. There are certainly some children who will benefit more than others from oxygen when the SpO2 is in the range of 90–92 %: those with very severe anaemia, severe heart failure, septic shock and acute neurological illness. These children will be less able to withstand moderately low oxygen levels than children with only lung disease.
Because the normal SpO2 range is lower at higher altitudes, it may be appropriate to only give oxygen for an SpO2 of 85 % or less to children living at an altitude above 2,500 m, if oxygen supplies are limited (e.g. when using oxygen cylinders and transport difficulties or cost limit supply) (Duke 2003). Oxygen concentrators, which provide continuous, unlimited oxygen, largely overcome this problem. If these are available, a universal threshold of 90 % SpO2 will be appropriate.
20.1.2 What to Do if the Child Does Not Improve or Deteriorates After Oxygen Is Given
It is very important that after starting oxygen therapy, the child is checked within 15–30 min to see if the treatment is working. In severely hypoxaemic children, correction may not be complete and clinical signs may remain, or the SpO2 may still be low. This does not mean that oxygen therapy has failed and should be abandoned. Other children will deteriorate rapidly or slowly despite receiving oxygen. There are a number of possible causes for a lack of response.
Oxygen delivery is inadequate, so check that:
Flow is occurring (hold the tubing close to your face to feel the flow).
There are no leaks from oxygen tubing.
The nasal prongs or nasal catheter are fitted correctly and not blocked.
If delivery is via an oxygen concentrator, the concentration of oxygen being delivered is adequate (>85 %).
There are other problems (see the WHO Pocket Book of Hospital Care for Children, Chapter 4) (World Health Organization 2005), such as:
Pleural effusion. Listen with a stethoscope for breath sounds on both sides of the chest; do a chest X-ray.
Pneumothorax. Listen with a stethoscope for breath sounds on both sides of the chest; do a chest X-ray.
Upper airway obstruction (e.g. from croup or a foreign body). Listen for stridor.
Bronchospasm (e.g. severe asthma). Listen with a stethoscope for wheeze.
Cyanotic heart disease or congestive heart failure.
Ventilatory failure. The child’s respiratory effort is inadequate; the child will have slow or shallow breathing and be lethargic.
If nasal prongs are being used at maximum flow and the child is still hypoxaemic, sometimes it is useful to give a second source of oxygen, if it is available, via an oxygen mask (ideally with reservoir bag) to increase the fractional concentration of inspired oxygen.
If a second source for mask oxygen is not available, an N-P catheter can give a higher fractional concentration of inspired oxygen than nasal prongs (but never use nasal prongs and an N-P catheter together).
20.1.3 Monitoring the Progress of Children on Oxygen
In most hospitals, the most appropriate form of monitoring will be regular checks with pulse oximetry on children who might need oxygen, those who are already on oxygen, those who have developed respiratory distress and those who show other clinical signs of deterioration. Oximetry can also be used to determine how long children need to be treated with oxygen. In severe pneumonia the duration of hypoxaemia may be anything from several hours to several weeks; the usual time is 2–5 days (Duke et al. 2000, 2002b). The duration of hypoxaemia may be longer at higher altitudes than at sea level for a similar severity of pneumonia (Weber et al. 1995).
Children who are receiving oxygen should be monitored clinically at least twice a day with pulse oximetry. Children in a stable condition should be tried off oxygen once a day to determine if they still require oxygen.
It is important to be aware that pulse oximeters provide no information on carbon dioxide concentration in the blood and thus no direct information on ventilatory efficiency. It is unlikely that a child who has normal oxygen saturation while breathing room air has impaired ventilation. However, once oxygen is administered, SpO2 can be maintained at normal levels despite severe hypercapnoea. In a child receiving supplemental oxygen, oximetry cannot be used to monitor the adequacy of ventilation. For children receiving oxygen, therefore, clinical monitoring of respiratory effort, respiratory rate and consciousness level is a better guide to the adequacy of ventilation. A child with inadequate ventilation will have slow or shallow breathing and be lethargic.
In a small hospital, any concern over the adequacy of ventilation should prompt efforts to ensure that the airway is clear and protected and that the patient is positioned to facilitate chest expansion (e.g. sitting in a semi-recumbent position of 20–30°, head up to reduce diaphragmatic splinting if there is abdominal distension, passing a nasogastric tube to deflate the stomach). Referral to a high-dependency area or intensive care unit should be arranged if CPAP or mechanical support is available.
All methods of oxygen administration need supervision by trained personnel to detect and manage complications appropriately. A nurse should check every 3 h that the prongs or catheter are in the correct position and not blocked with mucus, that all connections are secure, that the oxygen flow rate is correct, that the airways are not obstructed by mucus and that there is no gastric distension. Prongs or catheters should be removed and cleaned at least twice a day.
All severely ill children need regular monitoring of vital signs and general condition. Many deaths in hospitals occur overnight, often when monitoring is infrequent or absent. SpO2 is the most vital of clinical signs, so pulse oximetry is an invaluable, routine monitoring tool.
20.1.4 Trials Off Oxygen and When to Stop Oxygen
At least once each day, children in the ward who are clinically stable (have no emergency signs and SpO2 >90 %) should be disconnected from oxygen for 10–15 min and carefully examined for changes in clinical signs and SpO2, to assess whether supplemental oxygen is still required. Trials off supplemental oxygen are best done first thing in the morning, when there is likely to be adequate staff to observe the child throughout the day. If trials off supplemental oxygen are started in the late afternoon, low staff numbers overnight and the oxygen desaturation that sometimes occurs during sleep mean that there is a risk of hypoxaemia developing unrecognized overnight.
Children who have an SpO2 <90 % while still on oxygen or who are unstable or very unwell should not be given trials on room air.
Before a trial off oxygen, the SpO2 should be checked to determine if the trial is safe (i.e. SpO2 >90 %). The child should then be disconnected from the oxygen source and observed carefully to avoid any adverse complications of hypoxaemia. If severe hypoxaemia (SpO2 <80 %), apnoea or severe respiratory distress occurs, children should be immediately restarted on oxygen. Some children will become hypoxaemic very rapidly when they are taken off oxygen, and this is a marker of very severe disease and a high risk of death. Parents and nursing staff should be advised to watch the child to see if he/she develops cyanosis or severe respiratory distress.
Where oxygen supplies are ample, children should receive supplemental oxygen until their SpO2 on room air is 90 % or greater. If the SpO2 is 90 % or more after a trial on room air, they should remain off oxygen and the SpO2 should be rechecked after 1 h, as late desaturation can sometimes occur. Any child who appears to deteriorate clinically should have their SpO2 checked to determine whether they need oxygen. If bed space allows, children should not be discharged until their SpO2 has been stable at 90 % or more while breathing room air for at least 24 h, until all danger signs have resolved and until appropriate home treatment can be organized. This of course does not apply to children with cyanotic congenital heart disease, who have chronic hypoxaemia. For children with right to left intracardiac shunts (such as tetralogy of Fallot), oxygen will not be effective in relieving cyanosis or improving SpO2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


