Respiratory Distress Syndrome
Thomas N. Hansen and Samuel Hawgood
Neonatal respiratory distress syndrome is the most common cause of respiratory failure in the first days after birth, occurring in 1% to 2% of newborn infants. Until about 25 years ago, approximately 50% of infants with this condition died.1 Over the last three decades, improved methods of treatment have markedly reduced mortality, and the majority now survive.
Hyaline membrane disease (HMD) occurs mainly in prematurely born infants and is more common in whites than in blacks. It develops when an infant attempts to ventilate an immature lung with small respiratory units that inflate with difficulty and do not remain gas-filled between respiratory efforts. This behavior is due to rudimentary alveolarization of the lung and an inadequate amount of pulmonary surface-active material or surfactant. When surfactant is absent, the surface tension at the interface between alveolar gas and the alveolar wall is high, and the lung tends to become progressively atelectatic. This causes increasingly labored breathing and cyanosis as the volume of the lung decreases and the infant hypoventilates.
It appears to take only 1 to 2 days following birth for an immature lung to mature as it responds to the surge of glucocorticoids and β-adrenergic compounds released by the stress of delivery. Glucocorticoids increase surfactant synthesis, and β-adrenergic stimulation promotes its secretion. Also, in 1 to 2 days, structural changes occur in the lung. Thin-walled respiratory units develop, and the number of capillaries increases. With these changes, the signs and symptoms of respiratory distress subside. However, during this brief interval, lung damage may occur from the combined effects of pulmonary edema, ischemia, pulmonary air leaks, oxygen toxicity, and injury from barotrauma if high pressures are used to mechanically assist ventilation. As a consequence, HMD can result in chronic lung disease that may persist for weeks or months.
PATHOLOGY
At postmortem examination, the lungs from infants with neonatal respiratory distress are firm and airless. Atelectasis is striking on gross inspection; when the lungs are fixed in inflation, only the airways and a few alveolar ducts are air filled (Fig. 54-1). Diffuse atelectasis and dilated terminal bronchioles and alveolar ducts lined with a homogenous hyaline-staining material characterize the microscopic picture (Fig. 54-2). The hyaline membranes are plasma clots containing fibrin, other plasma constituents, and cellular debris. The small pulmonary arterioles appear constricted. There is congestion of pulmonary capillaries and veins and an increase in pulmonary water with dilation of the lymphatics.
Interstitial air leaks are common, and collections of air are often seen around small airways and vessels (eFig. 54.1  ). In some cases, the alveoli and hyaline membranes contain red cells. Electron microscopic examination shows degeneration of epithelial and endothelial cells and rupture of the basement membranes. If death occurs after 3 or 4 days of respiratory distress, the hyaline membranes are fragmented and numerous macrophages appear in the intra-alveolar spaces. The pulmonary interstitium is widened and filled with round cells and fibroblasts. After the first week, there is a proliferation of alveolar epithelial type II cells and capillaries. In severe cases, chronic changes occur, including metaplasia of the bronchiolar epithelium and interstitial fibrosis. These changes are discussed more completely in Chapter 59.
). In some cases, the alveoli and hyaline membranes contain red cells. Electron microscopic examination shows degeneration of epithelial and endothelial cells and rupture of the basement membranes. If death occurs after 3 or 4 days of respiratory distress, the hyaline membranes are fragmented and numerous macrophages appear in the intra-alveolar spaces. The pulmonary interstitium is widened and filled with round cells and fibroblasts. After the first week, there is a proliferation of alveolar epithelial type II cells and capillaries. In severe cases, chronic changes occur, including metaplasia of the bronchiolar epithelium and interstitial fibrosis. These changes are discussed more completely in Chapter 59.
When inflated with air, the lungs accept only 10% to 20% of the gas accommodated by normal lungs. If after full expansion the distending pressure is lowered, the amount of gas retained at each pressure is a smaller proportion of the maximum gas volume than in the lung from an infant without neonatal respiratory distress. This indicates that retractive forces are high in the air-filled lung with HMD. When distended with liquid, there is less difference in the pressure-volume relationships between the normal lung and the lung with HMD. This behavior was explained when Avery and Mead2 showed that post-mortem extracts of lungs of infants with the disease did not have a low surface tension when studied with a modified Wilhelmy balance. The surface tension–surface area characteristics of extracts of lung with and without neonatal respiratory distress are shown in Figure 54-3. The immature lung with an inadequate amount of surfactant in the alveoli tends to become atelectatic, producing increasing respiratory failure.
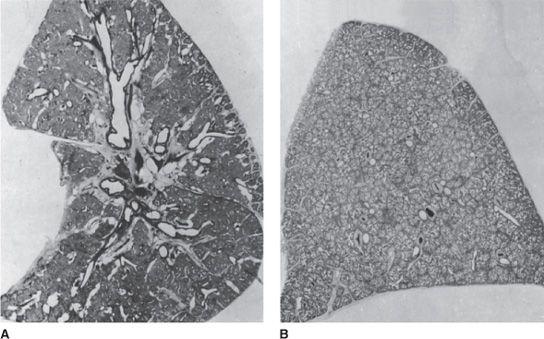
FIGURE 54-1. A. Longitudinal section of the left lung of a 1560-g infant, born at 30 weeks’ gestation, who died at 2.5 days of age from neonatal respiratory distress syndrome. The lung was expanded with air to a pressure of 40 cm H2O, then deflated to 10 cm H2O and fixed with the bronchus clamped. The airways are distended, and a few of the respiratory bronchioles are overinflated. Most of the alveolar ducts and alveoli are airless. B. Cross-section of the left upper lobe of a 1220-g infant, born at 29 weeks’ gestation without lung disease, who died at 1 week of age with a sudden, massive intraventricular hemorrhage. Inflation and fixation were identical to those used for the lung in A. Almost all of the alveolar ducts are filled with air, and the airways are not overdistended.
CLINICAL FEATURES
Some infants with HMD fail to expand their lungs at birth even with vigorous inspiratory efforts and have respiratory distress from delivery. Others initially inflate their lungs but develop progressive atelectasis and increasingly labored breathing in the first few hours of life. The characteristic clinical features of infants with neonatal respiratory distress are an expiratory grunt, tachypnea, intercostal and sternal retractions, and cyanosis.3 Grunting respiration is caused by a prolonged expiratory effort against a partially closed glottis. It is usually preceded by a strong inspiratory effort during which the intrathoracic pressure drops well below atmospheric pressure. During the prolonged expiration, intrathoracic pressure is maintained above atmospheric pressure. Infants do not grunt with every breath, and those with severe disease grunt most frequently. By maintaining a positive intrapulmonary pressure during most of the respiratory cycle, grunting probably helps prevent atelectasis. When not grunting, infants with HMD have small tidal volumes and a rapid respiratory rate. Apneic periods and irregularities of respiratory rhythm are common as the work of breathing increases and the infants become fatigued.
The large negative intrathoracic pressures generated as the infant attempts to inflate its lungs cause soft tissues of the chest cage to retract. These retractions are particularly notable in very small preterm infants with compliant chest walls. With severe respiratory distress, the lower sternum may be pulled in almost to the vertebral column by the forceful contraction of the diaphragm. Because the chest wall is so compliant and infants breathe primarily with the diaphragm, they often have paradoxical breathing movements. With labored inspirations, the chest wall is sucked in while the descent of the diaphragm increases lung volume in a cephalocaudad direction, encroaching on the abdominal cavity. Thus, as the chest caves in, its circumference becomes smaller while the abdominal circumference increases. This is accompanied by flaring of the alae nasal during inspiration. Breath sounds are diminished in intensity and have a harsh, tubular quality. Occasionally, there are fine rales, particularly in those infants born by cesarean section who may have excessive lung liquid. Cyanosis is an early sign and, as the disease progresses, may be present even when an infant breathes 100% oxygen.
As the lungs become more difficult to ventilate, the work of breathing increases, the infant tires, and arterial carbon dioxide tension rises. At the same time, the hypoxemia and diminished peripheral blood flow cause metabolic acidosis as lactic acid accumulates. With the development of acidosis, potassium leaves the cells and its concentration in serum rises, in some instances to very high levels.
Urine output is usually diminished early in the course of the disease, and the infants may become progressively edematous. Some in fants, especially very-low-birth-weight infants, have systemic hypotension, peripheral pallor, slow capillary filling, and hypothermia.
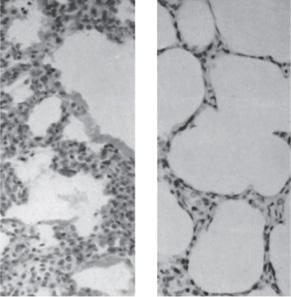
FIGURE 54-2. Left portion of the lung shown in Figure 54-1A (×100). Some of the alveolar ducts are inflated, but there are no true alveoli. The cells of the interstitial tissue appear to be crowded, but no inflammatory cells are present. The homogenous staining material lining the walls of the alveolar ducts are plasma clots (ie, hyaline membranes). Right lung shown in Figure 54-1B (×100). The interstitial tissue is thin. Although there are no true alveoli in this section, the total internal surface area is large, particularly compared with the lung in the left panel.
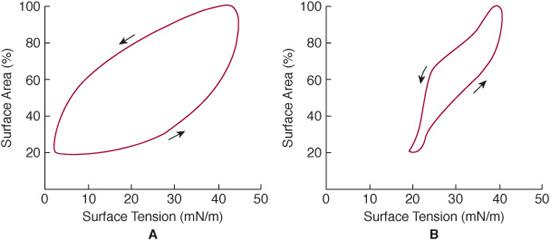
FIGURE 54-3. Surface tension–surface area diagrams of extracts of lung from an infant with neonatal respiratory distress (A) and a lung from an infant with normal lungs (B). These were obtained from a modified Wilhelmy surface-tension balance in which surface tension is continuously recorded as the surface area of the lung extract is reduced and increased. When the surface area is reduced to 20% (ie, equivalent to a lung changing from total lung capacity to residual volume), the surface tension of the extract from the lung with neonatal respiratory distress (B) is 20 mN/m, a high tension that if present at the surface of the alveolus would favor atelectasis. The surface tension at 20% surface area for the extract of normal lung is 3 mN/m (A).
In mild to moderate disease, respirations become increasingly difficult for about 48 hours, and after 72 hours of age, there is rapid improvement. Recovery is usually heralded by diuresis. Clinical improvement is accompanied by a rapid fall in pulmonary vascular resistance and a rise in systemic arterial pressure. In some infants, particularly the least mature with birth weights less than 1500 g, this may permit development of a large shunt from the aorta through a patent ductus arteriosus to the pulmonary artery. In these infants, recovery may be interrupted by the development of pulmonary edema. The symptomatic ductus arteriosus will usually require treatment with the prostaglandin inhibitor indomethacin or operative ligation (see Chapter 55).
Except during grunting, respiratory rate is rapid and tidal volumes are small. Minute ventilation is usually increased, but dead space ventilation is also increased so alveolar ventilation is low. Lung volume is low4 and tends to decrease further during the first 48 hours after birth in infants who are not being treated with positive end-respiratory pressure. As expected, lung compliance is low.5 Interestingly, airway resistance is increased. This increase occurs because noncartilaginous airways are dependent on surfactant for stability, because small airways are compressed by collections of interstitial fluid, and finally, because small airways are damaged by the large transpulmonary pressures needed to inflate the lung. The reduction in lung compliance and increase in airway resistance result in a marked increase in the work of breathing.
Evidence from nitrogen washout curves and expiratory flow volume curves suggests that infants with HMD have a marked maldistribution of ventilation. Surfactant deficiency is not uniform, and regions of the lung with adequate surfactant coexist with areas of surfactant deficiency. Compliance and resistance are normal in surfactant sufficient areas, while compliance is reduced and resistance is increased in areas of surfactant deficiency. During inspiration, most of the gas flow into the lung is distributed to the relatively normal lung units, with very little going to the abnormal lung units. In the poorly ventilated lung units, the oxygen supply from the atmosphere does not keep up with oxygen removal by the blood, so the alveolar partial pressure of oxygen (PO2) is markedly reduced. In response to the reduced alveolar PO2, blood vessels supplying the poorly ventilated lung units constrict and divert blood away from this part of the lung. Some of this blood is diverted to the more normal part of the lung, but most is diverted through right-to-left shunts. While these shunts can occur across the foramen ovale and ductus arteriosus early in the disease, for the most part, they occur in the lung through blood vessels that have developed prior to alveoli and through blood vessels supplying atelectatic alveoli. Therefore, the cause of much of the hypoxemia in infants with HMD is right-to-left shunt. However, the magnitude of the right-to-left shunt is determined by the size of the poorly ventilated compartment of the lung and by the oxygen tension in the alveoli in that compartment. In other words, the size of the right-to-left shunt is not fixed but is highly variable. If the alveolar oxygen tension in the poorly ventilated lung units can be increased by administering supplemental oxygen or by mechanical ventilation, much of the vasoconstriction can be relieved. With relief of vasoconstriction, the magnitude of the right-to-left shunt will decrease and arterial PO2 will increase.
The more normal parts of the lung in infants with HMD receive almost all of the ventilation, but because much of the right ventricular output is diverted through the shunt, these units receive a much smaller fraction of the total right ventricular output. These lung units are ventilated out of proportion to their perfusion and constitute a large physiological dead space.
RADIOLOGICAL FEATURES
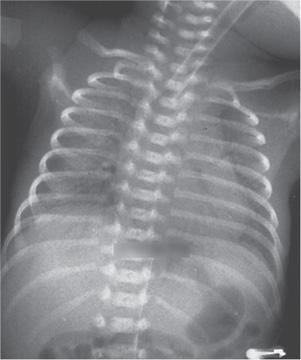
The radiographic appearance of the lungs in infants with neonatal respiratory distress is characterized by a diffuse reticulogranular pattern of increased density, usually uniform in distribution (Fig. 54-4) but occasionally more marked in the bases or on one side. The densities are due to miliary atelectasis and interstitial edema. Lung volume is small, and even radiographs taken after a maximal inspiration rarely show the diaphragm to be below the eighth to ninth interspace. The bronchial tree is clearly outlined by air against the poorly aerated lung—an air bronchogram. The heart is usually normal in size, although it often appears large because of the large thymic shadow and decreased lung volume. Often, diffuse atelectasis is not seen because the radiographic appearance of the lung can be markedly altered by treatment. Infants breathing against a positive pressure or being ventilated with intermittent positive pressure with positive end-expiratory pressure may have well-aerated lungs without air bronchograms. On the other hand, some infants with very severe disease may be unable to expand their lungs and have totally opaque radiographs in which even the heart borders are obscured. Later in the course of the disease, pulmonary edema, air leaks, or pulmonary hemorrhage can also affect the radiological appearance.
PATHOGENESIS
HMD is caused by atelectasis that develops from 3 interrelated factors: small respiratory units, a weak chest cage, and an amount of pulmonary surfactant that is inadequate to cover the internal surface of the lung.2 In the adult, the alveolar diameter is about 200 μm, and in the term infant, 100 μm. In the prematurely born infant, the alveolar ducts or acini are about 80 μm in diameter; there are no true alveoli. The diameter of the respiratory bronchioles of the prematurely born infant is relatively smaller than that of infants born at term. Because the respiratory units are curved, they are governed by the Laplace relationship, which states that the pressure difference (P) across a curved surface, needed to maintain a given radius (r), is inversely proportional to the radius and directly related to twice the surface tension (ST):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


