Background
Misoprostol is an effective agent for the induction of labor. Existing guidelines recommend oral misoprostol solution 25 μg every 2 hours. However, more research is required to optimize the use of oral misoprostol solution for the induction of labor.
Objective
The purpose of this study was to compare efficacy and safety of hourly titrated-dose oral misoprostol solution with static-dose oral misoprostol solution every 2 hours for labor induction.
Study Design
In this randomized controlled study, oral misoprostol solution was administered as (1) 20 μg hourly (≤4 doses) that was increased in the absence of regular uterine contractions to 40 μg hourly (≤4 doses) and then to 60 μg hourly (≤16 doses) or (2) 25 μg every 2 hours until active labor began (≤12 doses). A sample size of 146 women was planned with the use of a projected 95% rate for the primary endpoint (vaginal delivery within 24 hours) for hourly titrated-dose misoprostol and 80% rate for static-dose misoprostol every 2 hours. Safety outcomes included maternal morbidity and adverse neonatal outcomes.
Results
From December 2013 to July 2015, 146 women were assigned randomly to treatment. Demographic and clinical factors were similar between groups, except for age. Vaginal delivery was achieved within 24 hours in 47 women (64.4%) who received hourly titrated-doses of misoprostol solution and 48 women (65.8%) who received 2-hourly static-dose misoprostol solution ( P =1.00). Rates of vaginal delivery within 24 hours did not differ significantly between treatment groups for women who were nulliparous ( P =1.00) or who had postterm pregnancies ( P =.66), a Bishop score of ≤3 ( P =.84), or oxytocin augmentation ( P =.83). Cesarean deliveries were performed within 24 hours in 9 women who received hourly titrated-dose misoprostol solution and 2 women who received 2-hourly static-dose misoprostol solution ( P =.056). Pyrexia and meconium-stained liquor occurred more frequently with the hourly titrated-dose regimen.
Conclusion
The static-dose oral misoprostol solution every 2 hours has similar efficacy as hourly titrated-dose misoprostol solution but with fewer side-effects and lower complication rates.
Evidence that has accumulated over the years supports the use of oral misoprostol as a safe and inexpensive drug for labor induction. In 2012, the International Federation of Gynecology and Obstetrics (FIGO) recommended an oral dose of 25-μg misoprostol solution every 2 hours to induce labor, citing the 2011 World Health Organization (WHO) recommendations for labor induction. The WHO strongly recommended this regimen by rating the quality of evidence as moderate and including data from the 2006 Cochrane Review by Alfirevic and Weeks. The more frequent dosing may address the short half-life of misoprostol, which is reported to reach a C max of 300–800 pg/mL in approximately 14–30 minutes, with a terminal half-life of 20–40 minutes. A 2014 Cochrane Review of oral misoprostol for labor induction included an additional 19 studies. The authors confirmed the previous conclusion, stating that, if using oral misoprostol, then “the evidence suggests that the dose should be 20 to 25 μg” given every 2 hours. They added that “the evidence supports the use of oral regimens over vaginal regimens.” The study by Cheng et al, which compared hourly titrated-dose oral misoprostol with vaginal misoprostol, inspired us to explore a stepwise titration.
In our previous randomized clinical trial (RCT), we observed a 70% rate of vaginal delivery within 24 hours with the stepwise hourly titrated oral misoprostol solution regimen, which was greater than the 55% rate in women who were given the standard treatment of a dinoprostone vaginal insert ( P =.05). However, this vaginal delivery rate with titrated oral misoprostol solution was significantly lower than the 94% that was achieved by Cheng et al. Conversely, a simpler regimen, such as the FIGO-recommended static-dose misoprostol solution every 2 hours, would enable a more widespread use of this drug. Therefore, the objective of this study was to compare the efficacy and safety of hourly titrated-dose oral misoprostol solution, as described by Cheng et al, with the FIGO-recommended static-dose oral misoprostol solution every 2 hours for labor induction.
Materials and Methods
This open-label randomized trial was approved by the King Abdulaziz University Hospital (KAUH) institutional review board (KAUH study number 944-12). We enrolled all women who were admitted to KAUH (Jeddah, Saudi Arabia) for whom induction of labor was indicated by their attending obstetrician, who met the eligibility criteria, and who provided written informed consent. Inclusion criteria were (1) singleton live pregnancy, (2) ≥34 weeks gestation, (3) Bishop score ≤6, (4) intact membranes, (5) cephalic presentation, and (6) reassuring fetal heart rate. Exclusion criteria were (1) hypersensitivity to misoprostol, (2) previous cesarean delivery or other uterine surgery, (3) severe pregnancy-induced hypertension (abnormal liver function test results, urine protein>1 g/d, blood pressure 160/100 mm Hg), (4) total pregnancies ≥4, (5) multiple gestations, (6) uterine contractions, and (7) significant maternal cardiac, renal, or liver disease. Women were assigned randomly (1:1) into the treatment groups (hourly titrated-dose or static dose every 2 hours) with the use of computer-generated numbers. Allocation concealment was carried out by the use of opaque envelopes that were distributed by the obstetrics nurse. Labor management at KAUH is standardized and includes electronic fetal monitoring that is performed 1 hour before and 1 hour after the start of induction and is continued after the beginning of uterine contractions until delivery. Intramuscular or intravenous analgesia is given for pain relief during labor. Delivery is carried out by in-house staff, usually residents and senior residents under the supervision of the on-call consultant.
Oral misoprostol solution was administered as a 1-μg/mL solution prepared from a 200-μg misoprostol tablet (Cytotec; Searle Pharmaceuticals, Leicester, United Kingdom) dissolved in 200 mL water, as previously described. Cutting the tablets is difficult and imprecise; however, preparing a misoprostol solution allows precise dosing, and the misoprostol remains active in the solution for 24 hours. The oral misoprostol solution was prepared fresh for each woman, and the unused solution was discarded. In the hourly titrated-dose group, the regimen described by Cheng et al was used in the following manner: The starting dose was 20-μg (20 mL) oral misoprostol that was administered hourly for ≤4 doses; in the absence of regular uterine activity, the dose was increased to 40 μg (40 mL) hourly for ≤4 doses, and then to 60 μg (60 mL) for ≤16 doses. In the static-dose every 2 hours group, the recommended FIGO regimen was used in the following manner : Oral misoprostol solution 25 μg (25 mL) was administered every 2 hours for a maximum of 12 doses or until the onset of regular uterine activity. In both groups, no further misoprostol was given once regular uterine activity was observed. If contractions subsequently became inadequate, oxytocin was provided ≥2 hours after the last misoprostol dose. Regular uterine activity was defined as regular uterine contractions every 3–5 minutes and lasting ≥60 seconds. The primary outcome was successful labor induction, defined as vaginal delivery within 24 hours after treatment initiation. Secondary outcomes were rate of cesarean delivery and need for oxytocin augmentation. Safety outcomes included incidence of maternal morbidity and adverse neonatal outcomes. Uterine tachysystole was defined as >5 contractions in a 10-minute period without fetal heart rate changes. Uterine hyperstimulation was defined as hypertonic uterine contractions or uterine tachysystole that was associated with fetal heart rate abnormalities. To minimize bias, abnormal fetal heart rate tracings, uterine contractile abnormalities, and other intrapartum events were determined and managed by the in-house staff and not post-hoc by the researchers.
The use of a projected 95% rate of vaginal delivery within 24 hours for the hourly titrated-dose misoprostol regimen, as described by Cheng et al, and 80% rate for the recommended static-dose misoprostol regimen every 2 hours would require 73 women per group (alpha=.05 and 80% power). Our 70% rate of vaginal delivery within 24 hours for the stepwise hourly titrated-dose misoprostol solution regimen from our previous RCT was not used because the maximum cumulative dose in our study was 460 μg compared with 1120 μg in the study by Cheng et al, which might have contributed to our lesser rate of vaginal deliveries within 24 hours.
Analysis was performed on an intent-to-treat basis. The data were analyzed with the use of the Statistical Package for the Social Sciences (version 22.0; SPSS Inc, Chicago, IL). Dichotomous variables were compared between groups with χ 2 analysis or Fisher’s exact test, as warranted; continuous variables were compared with the use of the independent t -test ( P <.05 indicated statistical significance).
Results
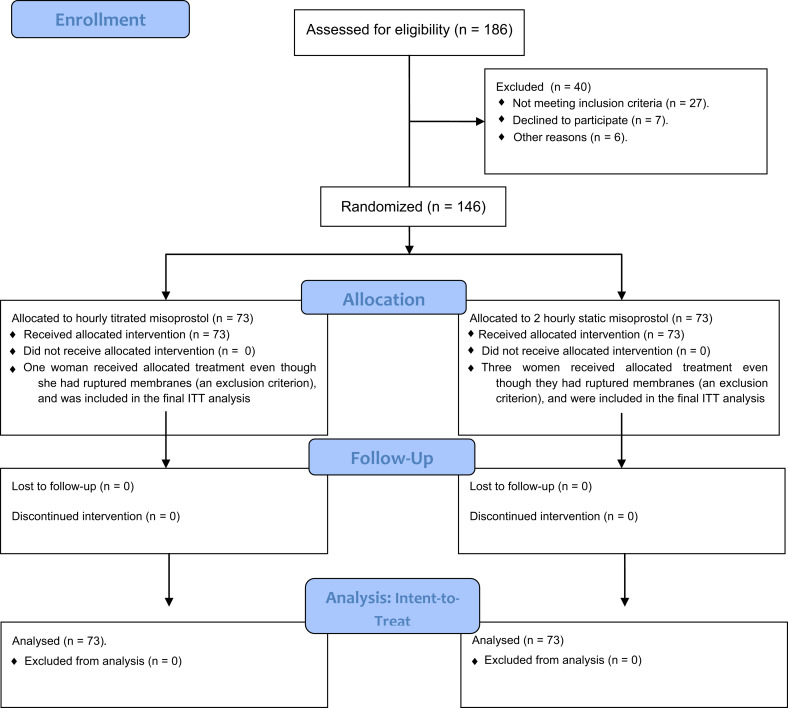
Of the 186 women who data were assessed for eligibility between December 2013 and July 2015, 146 women were assigned randomly to treatment (hourly titrated dose, 73 women; static-dose every 2 hours, 73; Figure ). Demographic and clinical factors were similar between groups, except for age ( Table 1 ). Patient age (mean±standard deviation) was 27.2±5.3 years (range, 17–42 years) in the hourly titrated-dose group and 29.3±5.1 years (range, 19–45 years) in the static-dose every 2 hours group ( P =.01). Postterm pregnancy was the primary indication for labor induction for 90 women (61.6%). Four women with premature rupture of the membranes at term were included in the study (titrated-dose group, 1 woman; static-dose group, 3 women). These 4 women with enrollment violations were included in the intent-to-treat analysis. Women in the hourly titrated-dose group received a median of 9 doses (range, 3–21); the median dose was 300 μg (range, 60–1020 μg). Women in the static-dose every 2 hours group received a median of 6 doses (range, 1–11); the median dose was 150 μg (range, 25–275 μg).
| Variable | Hourly titrated (n=73) | 2-Hourly Static (n=73) | P value |
|---|---|---|---|
| Age, y a | 27.2±5.3 (17–42) | 29.3±5.1 (19–45) | .01 |
| Gestation, wk a | 39.9±1.55 (34–42) | 39.7±1.49 (37–43) | .44 |
| Body mass index, kg/m 2 a | 32.6±6.4 (23.5–59.1) | 31.7±6.2 (19.6–44.9) | .42 |
| Nulliparous, n (%) | 40 (54.8) | 30 (41.1) | .13 |
| Bishop score ≤3, n (%) | 52 (71.2) | 57 (78.1) | .44 |
| Indication, n (%) | |||
| Postterm | 48 (65.8) | 42 (57.5) | .39 |
| Intrauterine growth restriction | 1 (1.3) | 7 (9.6) | .06 |
| Pregnancy-induced hypertension | 7 (9.6) | 4 (5.5) | .53 |
| Diabetes mellitus | 5 (6.9) | 4 (5.5) | 1.00 |
| Oligohydramnios | 2 (2.7) | 2 (2.7) | 1.00 |
| Premature rupture of membranes | 1 (1.3) | 3 (4.1) | .62 |
| Other | 9 (12.3) | 11 (15.1) | .81 |
Vaginal delivery within 24 hours was achieved in 95 women (65.1%) in the entire cohort, including 47 women (45 normal vaginal deliveries and 2 vacuum extractions) in the hourly titrated-dose group (64.4%) and 48 women (43 normal vaginal deliveries and 5 vacuum extractions) in the static dose every 2 hours group (65.8%; relative risk, 0.98; 95% confidence interval, 0.77–1.24; P =1.00; Table 2 ). Cesarean delivery was performed in 17 women (23.2%) in the hourly titrated-dose group: 11 women (64.7%) for fetal distress and 6 women (35.3%) for failure to progress. Cesarean delivery was performed in 6 women (8.2%) in the static dose every 2 hours group: 2 women (33.3%) for fetal distress and 4 women (66.7%) for failure to progress (relative risk, 2.83; 95% confidence interval, 1.18–6.77; P =.02). The indication of induction of labor in the 17 women who delivered by cesarean section in the hourly titrated-dose group was postterm pregnancy in 9 women, pregnancy-induced hypertension in 2 women, intrauterine growth restriction in 1 woman, oligohydramnios in 1 woman, diabetes mellitus in 1 woman, and other indications in 3 women. The indication of induction of labor in the 6 women who delivered by cesarean section in the static dose every 2 hours group was postterm pregnancy in 3 women; intrauterine growth restriction in 1 woman, oligohydramnios in 1 woman, and other indication in 1 woman. Similarly, cesarean deliveries were performed within 24 hours after treatment in 9 women (53%) in the hourly titrated-dose group but in only 2 women (33.3%) in the static dose every 2 hours group (relative risk, 4.5; 95% confidence interval, 1.00–20.11; P =.056). Proportions that achieved vaginal delivery within 24 hours did not differ significantly between treatment groups for women who were nulliparous ( P =1.00), who had postterm pregnancies ( P =.66), or who had a Bishop score ≤ 3 ( P =.84).
| Variable | Hourly misoprostol (n=73), n/N (%) | 2 Hourly misoprostol (n=73), n/N (%) | P value | Relative risk (95% confidence interval) |
|---|---|---|---|---|
| Delivered vaginally in ≤24 h | 47/73 (64.4) | 48/73 (65.8) | 1.00 | 0.98 (0.77–1.24) |
| Cesarean delivery | 17/73 (23.2) | 6/73 (8.2) | .02 | 2.83 (1.18–6.77) |
| Subgroups: Vaginal delivery in ≤24 h | ||||
| Parity: nulliparous | 21/40 (52.5) | 16/30 (53.3) | 1.00 | |
| Indication: postterm | 30/48 (62.5) | 29/42 (69.0) | .66 | |
| Bishop score ≤3 | 33/52 (63.5) | 38/57 (66.7) | .84 | |
| Oxytocin | ||||
| Yes | 39/54 (72.2) | 39/52 (75) | .83 | |
| No | 8/19 (42.1) | 9/21 (42.9) | 1.00 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


