Radiologic Imaging
Valerie L. Ward
Children and adolescents with gynecologic problems often need imaging examinations to determine the source of the pelvic pain, amenorrhea, structural anomalies, or heavy bleeding. Selection of the appropriate imaging examination depends on the acute nature of the presentation, the differential diagnosis, and the ease of the imaging examination for the age of the patient.
Ultrasound of the Female Pelvis
Ultrasound (US) is the imaging modality of choice for studying the pelvis in children and adolescents (1,2). In many children, US proves to be the only imaging modality needed (3). In children and young adults with acute lower abdominal pain, US has been shown to change the initial clinical diagnoses and change treatment decisions (4). While both transabdominal (TA) and transvaginal (TV) ultrasound examinations can be performed, the vast majority of US examinations performed in children and adolescents are TA. TA US does not utilize ionizing radiation and does not require sedation, even in uncooperative children (1,5). TA US provides a global view of the anatomy, shape, size, and echogenicity of the pelvic organs, and an analysis of the relationships between any structure and the uterus or ovaries (5,6). The kidneys are routinely imaged as a part of the gynecologic US examination, because congenital gynecologic abnormalities are frequently associated with congenital renal abnormalities (see Chapters 12 and 15). There are other instances when evaluation of the kidneys provides additional information about the impact of a gynecologic disorder on the child or adolescent; for example, a large pelvic mass that exerts mass effect onto the ureters can result in hydronephrosis.
The bladder is the pelvic organ that is routinely imaged first. The urine in the patient’s full bladder is used as an ultrasonic window to facilitate TA US imaging (1,7). The patient is asked to drink liquids prior to the US examination to fill her bladder (7). The degree of bladder distention (nearly empty, partially full, or full) impacts the extent to which the other pelvic organs are optimally visualized (6). If the patient cannot take liquids by mouth (e.g., a patient who is being kept in NPO status), then either an intravenous line can be established to hydrate the patient and fill the bladder or a bladder catheter can be inserted to fill the bladder directly. The bladder should be well filled but not overdistended (6). If a child cannot maintain a full bladder, then the TA US images will be suboptimal. Other causes for suboptimal TA US images are overlying bowel gas (i.e., disguised anatomy due to the overlying bowel gas) and obesity (6,8).
If an adolescent has been sexually active and can tolerate TV ultrasound, this modality can help elucidate preliminary findings on TA US (8). Images obtained with TV US are not limited by bowel gas or obesity, and it is helpful in patients who cannot maintain the full urinary bladder that is necessary for TA US (8). TV US is easy to perform, well tolerated by patients, and a rapid imaging technique (8). TV US utilizes high-frequency transducers that are placed in close proximity to the pelvic organs; therefore, it provides enhanced anatomic detail, higher-resolution images, and greater diagnostic confidence (8). For example, TV US has been shown to provide better demonstration of fluid in the endometrial canal, pyosalpinx, and tubo-ovarian abscess in patients with possible pelvic inflammatory disease (8). TV US is useful for the evaluation of anomalies of uterine configuration, a retroverted uterus that is not clearly imaged on TA US, and subtle tissue textural changes in an ovarian mass, a hemorrhagic cyst, or a torsed ovary (8). The disadvantages of TV US are that it is an invasive imaging technique and has a limited field of view (8). Thus, TA US should be performed first in adolescent patients (since TA US may obviate the need for further imaging investigation), and TV US utilized as an adjunct to TA US in the evaluation of the sexually active adolescent with a pelvic disorder (8).
Transperineal (TP) US imaging (also called translabial US imaging) is useful in female neonates, infants, and young children for demonstrating the anatomy of the urethra, periurethral soft tissues, anterior rectum, and distal gynecologic tract (9). The technique for TP US is as follows: a linear array transducer is used to image the perineum with the patient supine, in a modified lithotomy position (9). The transducer should be covered, preferably with latex, and a thick layer of gel is used between the transducer and perineum to minimize air artifacts (9). The midsagittal plane of the perineum is located by aligning the transducer with the pubic symphysis and posterior urethra, and this allows visualization of the entire length of the urethra, anterior wall of the rectum, base of the bladder, and vagina (9). Imaging slightly to the right and left of the midsagittal planes (i.e., the parasagittal planes) can be helpful for visualizing the distal ureter, for example, in a patient with a low ectopic ureteral insertion (9). TP US has been useful in girls for diagnosing and evaluating labial masses, foreign bodies, hydrocolpos, hydrometrocolpos, urogenital malformations, periurethral abnormalities, and presacral masses (2,6,9).
Doppler imaging with both color and pulsed wave techniques provides additional US information about the normal and abnormal vascularity associated with pelvic structures (1). Doppler can be performed with TA, TV, and TP US examinations.
Because the experience of the sonographer and/or radiologist who perform and interpret the TA US significantly impacts the quality of the images (6), an experienced pediatric radiologist is preferred for interpreting the imaging in children and adolescents (1). Sector and linear array US transducers are used for TA imaging of the pelvis, and the transducer with the highest frequency to maximize image resolution should be used (2,6). Multifrequency US transducers have widened the choices for scanning (1).
Increasingly, standard 2-dimensional (2D) US is being augmented and improved upon by 3-dimensional (3D) US or
volume sonography (10,11). A standard TA and TV US image is acquired as a single 2D tomographic slice (12). On the other hand, 3D US acquires a series of 2D images covering a volume from a patient that may be displayed in multiple planes and any orientation after the acquisition (12). When 3D US is acquired and displayed over time, it is known as “real-time 3D US” or “4D US,” and in obstetrics real-time 3D US allows for visualization of fetal movement (11,12). Three-dimensional US has been shown to be a problem-solving tool in selected gynecologic indications including the assessment of müllerian duct anomalies, the endometrium and uterine cavity, adnexal lesions, cornual ectopic pregnancies, infertility, and prenatal imaging (10,12,13). Investigators have shown that in the diagnosis of müllerian duct anomalies, the images obtained with 3D US were practically equivalent to those obtained with magnetic resonance imaging (MRI) examinations and had similar diagnostic accuracy (13). These investigators reported that limitations of 3D US were observed when the lower part of the uterus was studied and in the evaluation for septa, but stated that 2D US is also limited in the evaluation for septa (13).
volume sonography (10,11). A standard TA and TV US image is acquired as a single 2D tomographic slice (12). On the other hand, 3D US acquires a series of 2D images covering a volume from a patient that may be displayed in multiple planes and any orientation after the acquisition (12). When 3D US is acquired and displayed over time, it is known as “real-time 3D US” or “4D US,” and in obstetrics real-time 3D US allows for visualization of fetal movement (11,12). Three-dimensional US has been shown to be a problem-solving tool in selected gynecologic indications including the assessment of müllerian duct anomalies, the endometrium and uterine cavity, adnexal lesions, cornual ectopic pregnancies, infertility, and prenatal imaging (10,12,13). Investigators have shown that in the diagnosis of müllerian duct anomalies, the images obtained with 3D US were practically equivalent to those obtained with magnetic resonance imaging (MRI) examinations and had similar diagnostic accuracy (13). These investigators reported that limitations of 3D US were observed when the lower part of the uterus was studied and in the evaluation for septa, but stated that 2D US is also limited in the evaluation for septa (13).
Three-dimensional US images are displayed either as multiplanar images (presented in three planes perpendicular to each other) or as volume rendered (most commonly presented as surface-rendered images that display the surface of a volume, e.g., a view of the fetal face that is generally used to show expectant parents in fetal imaging practices and commercial enterprises) (11,14). Important advantages of 3D US are that the volume data can be acquired rapidly, stored, subsequently reviewed, and interpreted offline (when the patient has left the examination room) (11). Therefore, 3D US can potentially improve patient throughput and the efficiency of clinical practice; also, since the 3D volume of anatomy can be reconstructed offline and in any plane, 3D US is less operator dependent than 2D US (12,15). The overall use of 3D US in adult gynecology is increasing, especially as an adjunct to standard 2D US when 2D images may be abnormal or difficult to interpret and an accurate diagnosis is required (10,11). This modality is being used frequently for the evaluation of congenital anomalies of the reproductive tract (see Chapter 12). More studies and clinical experience are needed to determine the role of 3D US in adolescent gynecology, and currently 2D US continues to be the standard US imaging technique for evaluation (11).
Other Pelvic Imaging Modalities: Magnetic Resonance Imaging, Computed Tomography Scans, or Plain Film Radiography
Imaging modalities such as MRI, computed tomography (CT) scans, and plain film radiography are needed for appropriate evaluation of pediatric or adolescent gynecologic disorders such as congenital müllerian abnormalities, pelvic masses, and tumors (5). After an initial US examination is performed, the multiplanar capabilities of MRI can provide more detail about a structural abnormality of the female reproductive tract or provide longitudinal tumoral surveillance without the use of ionizing radiation (1,2). MRI considerations must take into account its accessibility and the need to sedate an uncooperative patient (1).
The use of CT scanning must take into account the radiation dose associated with imaging the radiosensitive tissues in the pediatric and adolescent pelvis, and thus low-radiation-dose CT scan protocols should be used when imaging the pelvis (1). Based on this added risk, we rarely utilize CT for the evaluation of ovarian masses unless there is concern for malignancy. The fast scanning technique of modern CT scanners often lessens the instances when patient sedation is actually required. CT scans can complement the findings of an initial US examination by better delineating the internal contents of pelvic masses, such as fat tissue and calcification in a teratoma, or the extent of a pelvic inflammatory process or neoplasm beyond the field of view of the pelvic US (1,2,7). The female pediatric pelvis has a paucity of fat and the pelvic organs are small in size; therefore, CT scans should be performed with oral contrast material to opacify the rectum to better determine whether adjacent pelvic structures are involved in an inflammatory process or affected by a pelvic neoplasm (2,7).
Plain radiographs are low in resolution and, as in the case of CT scans, utilize ionizing radiation. Yet, plain radiographs may be helpful in demonstrating calcification in a pelvic mass, a fecalith in a child with appendicitis, bowel displacement by a pelvic mass, or bowel obstruction by a pelvic inflammatory process (2).
Normal Ultrasound Images of the Uterus, Endometrium, and Ovaries
Uterine Size and Morphology
US imaging can provide an accurate assessment of uterine and ovarian morphology and size for the diagnosis and evaluation of pediatric and adolescent gynecologic disorders (16,17). The formula that is frequently used for uterine and ovarian volume calculations is the simplified formula for a prolate ellipsoid: longitudinal diameter × anteroposterior diameter × transverse diameter × 0.5233 (7,16,18,19,20,21). Uterine volume is statistically significantly higher in neonates aged 0 to 1 month than in infants aged 1 to 3 months (20). In the first month of life, the uterine volume declines, and this decline in volume is a result of the withdrawal of maternal estrogens after birth (20). After age 2 months, US has shown that the uterine volume remains low and has a more constant size until the child reaches age 7 (16,17,20).
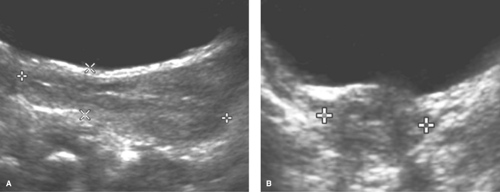
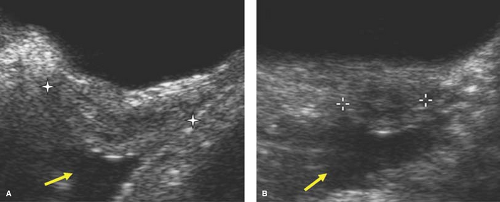
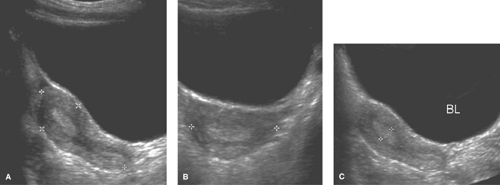
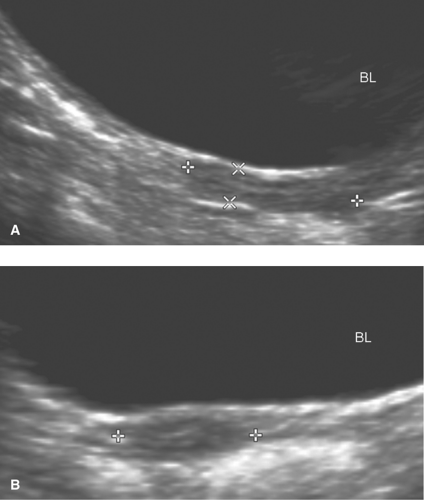
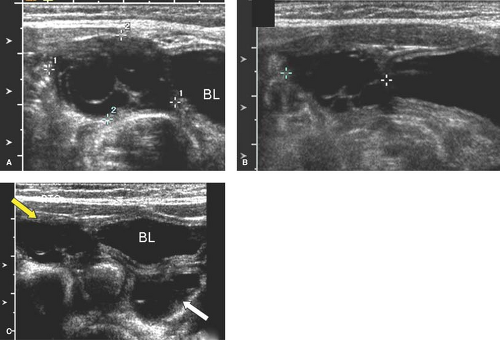
The uterine morphology on US also changes throughout childhood (see Figs. 23-1, 23-2, and 23-3) (16,17). Over time, the uterine shape increases such that the corpus gradually becomes larger than the cervix (17). Specifically, US of normal prepubertal girls has shown that the relationship of the cervix to corpus is 2:1 (so that the uterus has an “inverse” pear-shape morphology), and in postpuberal girls the relationship of the cervix to corpus changes to 1:2 (16,17). After age 7, there is a measurable age-related increase in uterine diameters and volume, and in older girls the uterus gradually reaches its typical adult pear-shape morphology (16). Investigators have shown that normative US data for the uterine and ovarian growth are related to chronologic age, bone age, and pubertal stage (19,20). At puberty, US measurements of uterine growth show a progressive increase (20,22) (see Table 23-1). If a child is exposed to high-dose radiation therapy and the uterus is in the radiation field, then the
uterus may not develop to its adult potential (see Fig. 23-4; see also Chapter 29).
uterus may not develop to its adult potential (see Fig. 23-4; see also Chapter 29).
Table 23-1 Normal Age-Related Uterine Diameters and Volumes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Normal Variability in Endometrial Thickness and Appearance
The US appearance of the normal endometrium in the adolescent can be correlated with cyclic changes, and US can detect subtle changes in the endometrium (23,24). TV US more clearly depicts the endometrial–myometrial junction of the uterus than does TA US (24,25,26). The endometrium is best visualized on the longitudinal images of the uterus, because a greater portion of the endometrium is included in this plane than in the transverse plane (23,26). The endometrial stripe thickness is usually measured from echogenic border to echogenic border across the endometrial lumen (25). During menstruation, the endometrium appears as a thin, continuous echogenic line measuring 1 to 4 mm in thickness (25,26). Intraluminal blood or sloughed endometrial tissue can be visualized in the endometrial lumen (25). During the early proliferative phase of the menstrual cycle, the endometrium becomes thicker (5 to 7 mm) and more echogenic relative to the myometrium (25). In the late proliferative phase (periovulatory phase), the endometrium develops a multilayered appearance and may measure up to 11 mm in thickness (25). During the secretory phase of the menstrual cycle, the endometrium becomes even thicker (7 to 16 mm) and more echogenic, and may even have increased posterior acoustic enhancement (25). The maximal endometrial thickness is seen on US during the midsecretory phase (25). A limitation of US imaging of the endometrium is that the endometrium of a retroflexed uterus may not be adequately visualized (23).
Ovarian Size and Appearance
On US, ovarian volume remains unchanged during the first 2 years of life and shows only a small increase before puberty (17,20). During puberty, there is a steep increase in ovarian volume (17). In the second decade of life, the mean ovarian volume is 7.8 mL with a range of 1.7 to 18.5 mL (27,28) (see Table 23-2).
In the premenarchal girl between ages 2 and 12, the ovary has a heterogeneous (rather than a homogeneous) US appearance (7). This heterogeneous appearance is due to variable-sized follicles or cysts within the premenarchal ovary in an otherwise healthy girl and does not suggest a pathologic diagnosis (7). The maximum follicular diameter in normal girls in early childhood is 7 mm (21).
Table 23-2 Normal Ovarian Volume Measurements with Age | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Ovarian Cysts
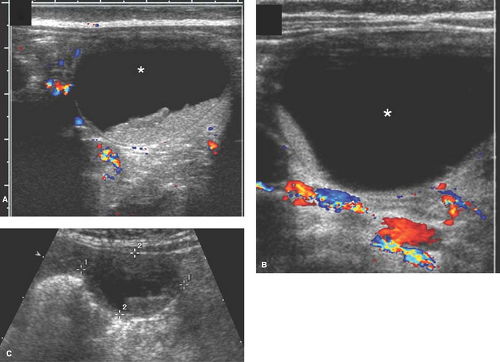
The US appearance of a cyst varies based on whether the cyst is uncomplicated (simple) or complicated (torsed or hemorrhagic) (29). On US, a simple ovarian cyst (see Chapter 21) is round or oval, well marginated (with imperceptible walls), located within the borders of normal-appearing ovarian parenchyma, and anechoic (i.e., does not contain internal echoes) (29,30). It does not contain fat, calcium, or septations (30). A simple cyst also has increased sound beam through-transmission.
Newborn girls can have cysts due to maternal stimulation from placental (human chorionic gonadotropin [HCG]) or fetal hormones (follicle-stimulating hormone [FSH]), and these cysts may not involute with time (31,32,33,34). Hence, small follicular cysts are a normal finding in the neonatal ovary (see Fig. 23-5) (29,35). Larger neonatal cysts may be diagnosed incidentally on prenatal US or postnatally in a neonate who presents for a US with a palpable mass or for postnatal US follow-up of a prenatal cyst diagnosis (29).
Neonatal ovarian cysts that do not resolve spontaneously may have complications (36). On US, a complicated neonatal ovarian cyst usually contains a fluid-debris level, retracting blood clot, septations, and an echogenic wall from dystrophic calcification associated with infarction, and may be associated with ascites from cyst rupture (29). A hemorrhagic neonatal ovarian cyst (see Fig. 23-6) usually results from in utero torsion and is almost always associated with infarction (29). A fluid-debris level on
US is a specific sign of cyst torsion in neonates and pre- and postpubertal girls (29,37).
US is a specific sign of cyst torsion in neonates and pre- and postpubertal girls (29,37).
Ovarian cysts in older girls and adolescents include simple cysts (Fig. 23-7), corpus luteum cysts (Fig. 23-8), paraovarian cysts, broad ligament cysts (Fig. 23-9




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree