Fig. 6.1
Cartoon diagram highlights the anatomic site for tumor origin of uterine sarcoma
Stage | Surgical-pathologic findings |
|---|---|
Leiomyosarcomas and endometrial stromal sarcomas | |
I | Tumor limited to the uterus |
IA | Tumor Less than or equal to 5 cm |
IB | Tumor More than 5 cm |
II | Tumor extends beyond the uterus, within the pelvis |
IIA | Tumor involves adnexa |
IIB | Tumor involves other pelvic tissues |
III | Tumor invades abdominal tissues (not just protruding into the abdomen) |
IIIA | One site |
IIIB | More than one site |
IIIC | Metastasis to pelvic and/or para-aortic lymph nodes |
IV | |
IVA | Tumor invades the bladder and/or rectum |
IVB | Distant metastasis (excluding adnexa, pelvic, and abdominal tissues) |
Carcinosarcomas | |
I | Tumor confined to the corpus uteri |
IA | Tumor limited to endometrium or invades less than half myometrial invasion |
IB | Tumor invades one-half or more of the myometrium |
II | Tumor invades stromal connective tissue of the cervix but does not extend beyond the uterus |
IIIA | Tumor involves s erosa and/or adnexa (direct extension or metastasis) |
IIIB | Vaginal involvement (direct extension or metastasis) or parametrial involvement |
IIIC | Metastases to pelvic and/or para-aortic lymph nodes |
IV | The bladder and/or bowel mucosa and/or distant metastases |
IVA | Tumor invades bladder mucosa and/or bowel (bullous edema is not sufficient to classify a tumor as T4) |
IVB | Distant metastasis (includes metastasis to inguinal lymph nodes, intraperitoneal disease, or the lung, liver, or bon e. It excludes metastasis to para-aortic lymph nodes, vagina, pelvic serosa, or adnexa) |
Adenosarcomas | |
I | Tumor limited to the uterus |
IA | Tumor limited to the endometrium/endocervix |
IB | Tumor invades less than half the myometrium |
IC | Tumor invades one-half or more of the myometrium |
II | Tumor extends beyond the uterus, within the pelvis |
IIA | Tumor involves adnexa |
IIB | Tumor involves other pelvic tissues |
III | Tumor involves abdominal tissues |
IIIA | One site |
IIIB | More than one site |
IIIC | Metastasis to regional lymph nodes |
IV | |
IVA | Tumo r invades bladd er or rectal mucosa |
IVB | Distant metastasis |
For past 20 years, molecular studies provided many new findings of the different genomic and genetic alterations in uterine sarcomas. These findings help us to better understand the complexity and relationship between normal and tumor cell types. However, the primary causes of uterine sarcoma remain largely unknown. In this chapter, we review the current literature on the clinical and pathological presentation and focus on the common and early molecular alterations in each of the uterine sarcoma types. In addition, we will provide some insights into the molecular biology, potential diagnostic biomarkers, and the tumorigenesis of uterine sarcomas, as well as their possible origin.
Uterine Carcinosarcoma
Clinical Features
Uterine carcinosarcoma (UCS), also referred to as “malignant mixed Müllerian tumor (MMMT) ,” accounts for almost half of all uterine sarcomas and 5 % of malignant uterine tumors [5]. Although the majority of UCSs appear at advanced ages (~50–70 years, with a median age of 65 years), a small number has been reported in patients under 40 years old. The symptoms are similar to endometrial carcinomas, with vaginal bleeding, pelvic mass, and lower abdominal pain. The incidence is increased in patients with an increased exposure to estrogen and pelvic radiation. As reported, patients treated with tamoxifen are eight times more likely to have UCS, and up to 30 % of patients with UCSs have a history of pelvic irradiation [6]. Patients with UCS usually present with extrauterine spread (41 % in stages III and IV) [7]. The serum level of CA125 is also elevated in most cases especially in extrauterine disease and deep myometrial invasion. The 5-year disease-specific survival is 47 % for stage I, 35 % for stage II, 22 % for stage III, and 10 % for stage IV [7].
Pathological Features
Grossly, UCSs are typically large, bulky, polypoid masses, filling the uterine cavity and prolapsing through the cervical canal with deep infiltration into the myometrium (Fig. 6.2). The cut surface often shows areas of hemorrhage, necrosis, and cystic change and frequently extends beyond the uterus.
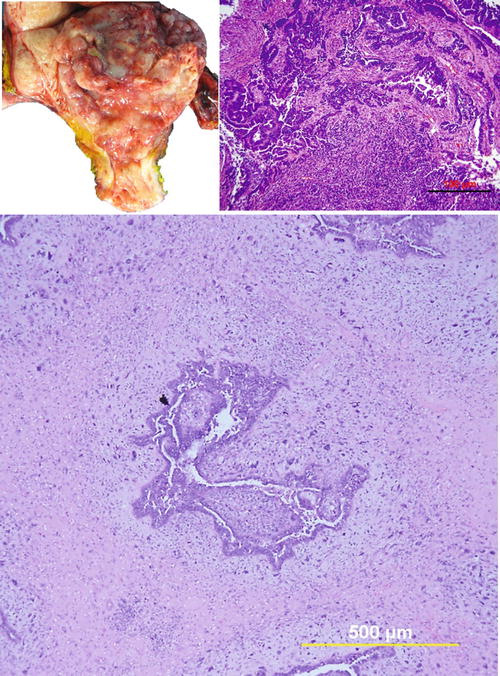

Fig. 6.2
Uterine carcinosarcoma. (a) Photomacrograph illustrates typical gross appearance of polypoid growth pattern of carcinosarcoma. (b) and (c) photomicrographs show biphasic tumor sections containing malignant epithelial component (high-grade serous carcinoma) and mesenchymal component (undifferentiated sarcoma)
Microscopically, UCSs are biphasic tumors, which include epithelial and sarcomatous components. The epithelial component is usually high grade (Fig. 6.2). The most common epithelial component is serous (two-thirds of cases) followed by endometrioid with other epithelial components including clear cell, squamous, and undifferentiated carcinoma. Most cases (72 %) consist of a single type of epithelial component, and the remaining (28 %) show 2–3 mixed epithelia. For the sarcomatous component, 80 % of the cases show high-grade sarcoma. The most common histological type is undifferentiated with areas of leiomyosarcoma, rhabdomyosarcoma, chondrosarcoma, osteosarcoma, and liposarcoma. The majority (67 %) reveal only one type of sarcoma, while the rest show multiple histologic differentiations [8, 9].
Immunohistochemistry and Genetic Aberrations
Due to the biphasic nature, the immunophenotypes are slightly different. For example, the epithelial component shows diffuse immunoreactivity for epithelial membrane antigen (EMA ) and cytokeratin , whereas the sarcomatous component can be positive for cytokeratin , but is usually patchy or focal. In contrast, sarcoma is positive for vimentin and other mesenchymal markers depending on the differentiation, such as desmin and caldesmon (smooth muscle differentiation), myogenin, and MyoD1 (rhabdoid differentiation). Diffuse immunoreactivity for P53 was firstly reported in 30 % (5/17) of UCSs, and there was no disparity between the epithelial and sarcomatous components [10]. Recent s tudies have confirmed that P53 is commonly overexpressed in UCSs with a positive rate ranging from 30 % to 80 %. Overall, 70–80 % of cases have a concordant immunostaining pattern for P53 in carcinoma and sarcoma. The MDM2 oncogene encodes a protein that binds to and inactivates the p53 gene product. MDM2 overexpression is found in 17 (4/23)–26 % (11/43) of UCSs [11, 12]. P16 overexpr ession is very common in UCSs, and it was reported to be as high as 96.7 % and 86.7 % of carcinoma and sarcoma, respectively [12–15]. Other gene mutations and dysregulation in UCS include but are not limited to TGF-β, Rb, HER-2, VEGF, ERβ, CATs, β-catenin, COX-2, and PTEN [16–22]. Notably, ERα, PR, IGF1R, and CD10 are often downregulated or completely lost in sarcoma [23]. A study examined the expression of 39 epithelial to mesenchymal transition (EMT)-related genes. Acquired markers of EMT were found to be upregulated, and attenuated markers of EMT were downregulated in UCSs. High expression of phospho-SMAD2/3 (p-SMAD2/3 ) indicated that TGF-β seems to play a major role in UCS. In the sa me study, chromosomal gains at 19q13, which includes the TGFB1 locus, were also identified by chromosomal Gene Set Enrichment Analysis (GSEA) and comparative genomic hybridization (CGH) microarrays in UCS [24]. In a ddition, upregulation of the Akt/β-catenin and Rb pathway may be essential for the establishment and maintenance of phenotypic characteristics of UCSs through the regulation of E-cadherin mediated by the transactivation of the Slug gene [25].
Global genomic analysis by comparative gen omic hybridization (CGH) and fluorescence in situ hybridization (FISH) in a series of 30 carcinosarcomas revealed that chromosomal gains (85 %) are more common than losses (30 %) in UCSs. Chromosomal amplification is frequently observed on chromosome 8q (42 %) and 20q (70 %). In these regions, c-myc (8q24.12) and ZNF217 (20q13.2) amplification can be identified in 78 % and 87 % of UCSs. Amplification of ZNF217 was mostly seen in both tumor components, whereas amplification of c-myc was observed less often in the sarcomatous than in the carcinomatous component [26]. These two genes correlate to d istant metastases and poo r prognosis [26, 27].
Theories of Tumor Origin
There are several hypotheses for the cell origin of UCS, including (1) monoclonal stem cell combination (origin from a common cell origin), (2) tumor collision (origin from two distinct malignant cell populations), and (3) composition (origin from metaplastic transformation from one neoplastic cell type) (Fig. 6.3). An early study illustrated a conversion of carcinomatous cells to sarcomatous cells using four clonal cell lines from ovarian carcinosarcoma [28]. An in vitro experiment on the EMTOKA cell line (a human UCS cell line) found that the same intermediate filament (IF), HER-2, P53 expression, and the ultrastructure characteristics can be found in EMTOKA and its clones [29]. These two studies support the monoclonal theory that the epithelial component and sarcomatous component may be derived from a common stem cell. A recent study examined the panel of immunoreactivity for MLH1, MSH2, MSH6, PTEN, P53, β-catenin, and cyclin D1 in 40 UCSs. They found that expression patterns of P53, MSH2, and MSH6 corresponded between the epithelial and mesenchymal components [30]. Furthermore, the two components also harbored similar chromosomal aberrations [26]. The presence of similar immunohistochemistry and genetic aberration patterns in both histological components in most UCSs are evident in favor of the monoclonal theory for either stem cell origin or composition theory due to the metaplastic transformation, whereas presence of different types of epithelial or sarcomatous components within one tumor can be better explained by the stem cell theory. It seems that some observations in favor of the biclonal origin theory will require the examination of additional cases and studies [31–33].
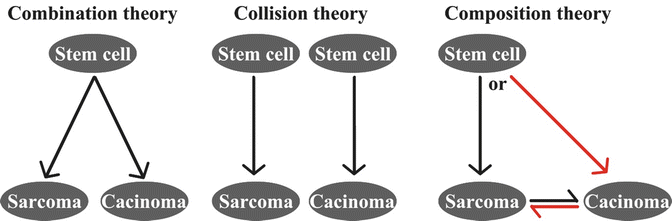

Fig. 6.3
Proposed mechanisms for tumorigenesis of uterine carcinosarcomas
Occasionally, collision tumors can be se en in clinical cases. For example, Jin et al. found that one of the 15 UCSs was probable collision tumors [33]. PAX8 is a useful immunohistochemical marker for these epithelial neoplasms of gynecologic origin. A study shows that the epithelial component strongly expressed PAX8 in 97 % of (36/37) the tumors, but only 27 % (10/37) mesenchymal component showed PAX8 expression in 27 cases (10/37) with variable expression [34].
Unfortunately, the precursor lesion for UCS remains unknown, and most cases are diagnosed in their invasive form.
Leiomyosarcoma
Clinical Features
Leiomyosarcoma (LMS) is the second most common type of uterine sarcoma, accounting for ~1–2 % of uterine malignancies [35]. According to the Surveillance, Epidemiology, and End Results (SEER) database, the incidence of uterine sarcomas from 1979 to 2001 was 0.36 per 100,000 women/year and may be increasing among women in the United States [2]. Most tumors were stage I (68 %), whereas stages II, III, and IV tumors represented 3 %, 7 %, and 33 % of cases respectively [36]. This disease is prevalent in postmenopausal women with the mean age of 60 years old. Signs and symptoms include abnormal vaginal bleeding (56 %), palpable pelvic mass (54 %), and pelvic pain (22 %). Preoperative distinction between benign and malignant smooth muscle tumors remains a challenge in the diagnosis. In hysterectomy for benign uterine smooth muscle tumors, 1/800 cases turns out to be leiomyosarcoma. A small proportion of tumors are identified as malignancies either through manifestations of their rupture (hemoperitoneum) and/or extrauterine extension or metastases. Interestingly, the development and progression of LMS seem not to be associated with hormones. Rather, histories of hereditary retinoblastoma or prior pelvic radiation are considered as the main risk factors.
Pathological Features
Grossly, LMSs are mainly a solitary uterine mass. They are usually large with a mean diameter of 10 cm (only 25 % are <5 cm). The cut surface is typically soft, yellow or tan, fleshy, necrotic, and hemorrhagic with irregular and infiltrating borders (Fig. 6.4).
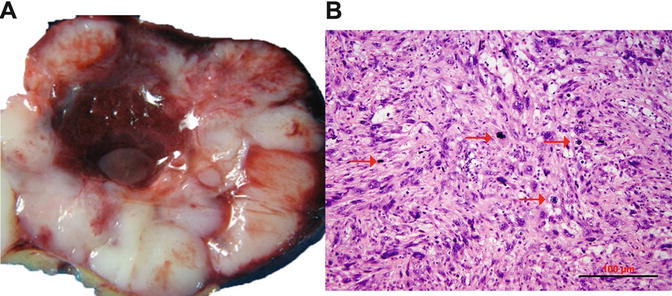

Fig. 6.4
Uterine leiomyosarcoma. (a) Gross appearance of leiomyosarcoma, characterized by tan and fleshy cut surface with hemorrhage and true tumor necrosis. (b) Histology section shows hypercellular spindle cell proliferation, prominent cytologic atypia, and brisk of mitoses
Most LMSs are spindle cell and hypercellular. Tumor cells show moderate to severe nuclear atypia and a high mitotic rate [generally exceeding 10 mitoses per 10 high-power fields (HPFs)]. Tumor necrosis (coagulated necrosis) can be seen in most cases. Epithelioid and myxoid LMSs, however, are rare variants with mild to moderate nuclear atypia, and the mitotic rate is often <5/10 HPF [37]. In clinical practice, the diagnosis of LMS can be sometime problematic due to a wide spectrum of histologic features shared with other uterine smooth muscle tumors (USMTs) as listed in Table 6.2. In a review of 356 cases with an original diagnosis of LMSs, only 72.7 % cases (259/356) could be confirmed as malignant tumors, whereas 27.3 % (97/356) were reclassified as benign or leiomyoma variants [35].
Leiomyoma variants with mimic malignancy |
Atypical leiomyoma (leiomyoma with bizarre nuclei) |
Smooth muscle tumors of uncertain malignant potential (STUMP) |
Cellular leiomyoma |
Mitotically active leiomyoma |
Myxoid leiomyoma |
Epithelioid leiomyoma |
Leiomyoma with massive lymphoid infiltration |
Smooth muscle tumors with unusual growth patterns |
Disseminated peritoneal leiomyomatosis |
Benign metastasizing leiomyoma |
Intravascular leiomyomatosis |
Lymphangioleiomyomatosis |
Immunohistochemistry and Genetic Aberrations
LMSs usually express smooth muscle markers such as smooth muscle actin (SMA), desmin, h-caldesmon, and histone deacetylase 8 (HDCA8). Only ~20–30 % cases express ER and PR [39–42]. A significantly higher level of cell proliferation (illustrated by a high Ki-67 index) is present in the majority of LMSs [43–47]. Recently, P16 has been discovered to be a new biomarker for LMS identified through global gene profile analyses [48]. Emerging data showed that ~71–100 % of LMSs are strongly and diffusely immunoreactive for P16, and therefore, it may be a useful adjunct immunomarker for distinguishing between benign and malignant uterine smooth muscle tumors [40, 43, 45, 49–56]. Additionally, PTEN, FASCIN, pAKT, pS6RP, P4EBP1, and β-catenin positive can be seen in most LMS [39, 57–60]. Mutation and overexpression of P53 have also been described in a significant number of LMSs (~20–43 %) but not in ULMs [46, 61–66] (Fig. 6.5). None of these markers, however, are absolutely discriminatory in the differential diagnosis between LMS and its potential mimics.
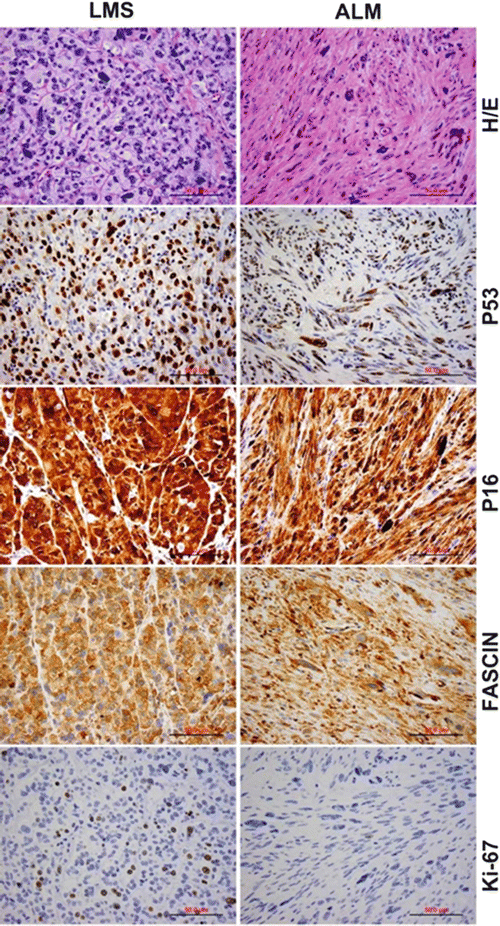

Fig. 6.5
Side-by-side comparison of leiomyosarcoma (LMS, left) and atypical leiomyoma (ALM, right) by histology and immunohistochemistry
Based on gene profile analysis, many cell cycle genes, oncogenes, and transcription factors are dysregulated and may be related to LMS tumorigenesis (Table 6.3) [48, 67–71]. In addition, LMSs present complex numerical and structural chromosomal aberrations, including frequent losses of 10q (where PTEN harbored), 12q, and 13q. A gain of 17p and losses of 2p and 16q are occasionally observed [72–77].
Function | Genes |
|---|---|
Upregulated | |
Cell cycle regulation | CDKN2A, CDK4, CDKN2A, CCND1, CCND3, CKS2, FOXM1, PTTG1, PRC-1, UBE2C, COPS3, MDM2 |
Cell homeostasis | GRIA2, NPTX2, CRABP2, POPDC2, ST5, TOP2A |
Cell structure | ACTC1, DIAPH3, DCX, COL5A2, COPS3, THBS2, PLP1 |
Signal transduction | MAP3K8, PIK3R1, IL17B, TSPAN31, SPP1 |
Growth factors | IGF1, IGFBP5, TGFB3 |
Transcription factors | E2F1, RB1, GLI1 |
Proteinases | MMP9, CAPN6 |
The actin cytoskeleton | CALD1, SLMAP, DMD, ACTG2, CASQ2, CFL2, MYLK, LPP |
Organ development | ADD3, ANTXR, FLJ39632, TCF4, FBN1, SNAI2, SPRY1, XPOT, FSTL1 |
Downregulated | |
Metabolism | ALDH1A1, ALDH1B1 |
Cell cycle and structure | CDKN1A, DPT, KRT19, CNN1 |
Cell homeostasis | TNXB |
Oncogenes | Mutations in KIT, MED12, IRF1 |
Signal transduction | MAP3K5, RNASE4 |
Theories of Origin
The pathogenesis of uterine LMS is poorly understood. It is generally believed that LMSs arise de novo [78]. Lately, this theory is being challenged as recent studies show that some uterine LMSs may be related to preexisting ULMs [79–81]. For example, one study shows that there are benign-appearing tumor components, defined as “leiomyoma-like” (Fig. 6.6), within LMS. These leiomyoma-like areas have fewer distinctive genomic alterations compared to their truly malignant counterpart. Furthermore, a high-density and chip-based analysis by CGH illustrates some different but partially shared genetic alterations between “leiomyoma-like” and fully malignant areas within one tumor mass. The findings suggest that some uterine LMS may arise from a preexisting benign uterine smooth muscle tumor [82].
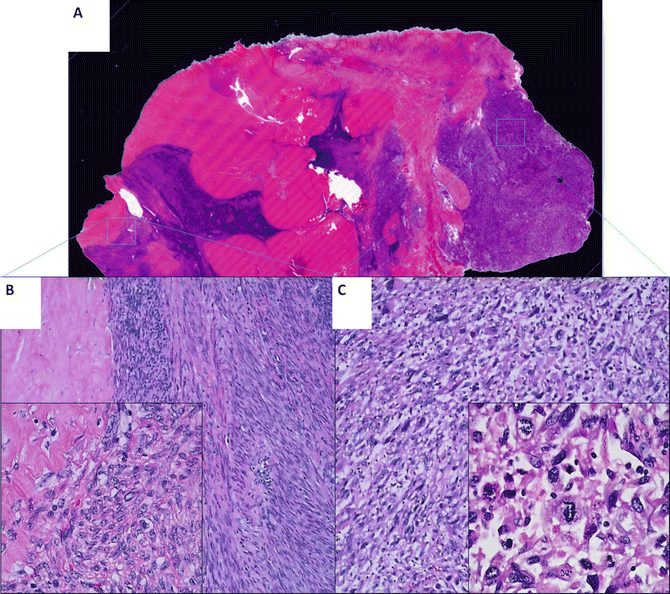

Fig. 6.6
A large leiomyosarcoma with leiomyoma-like area. A full mounted tissue section (a) with benign leiomyoma-like area, showing spindle cell tumor with minimal cytologic atypia, low mitotic count, and hyalinized change (b) and area of fully malignant area, characterized by hypercellular spindle cell proliferation with high-grade cytologic atypia and frequent mitoses/atypical mitosis (c)
A mouse model utilizing a deletion of PTEN alleles results in widespread smooth muscle cell hyperplasia and a high rate of abdominal LMSs with a very rapid onset and elevated incidence (~80 %) of LMS. Apparently, the AKT-mTOR pathway plays a critical role for smooth muscle cell transformation and LMS genesis [83].
Atypical leiomyoma (ALM ) , as defined by Stanford researchers [84], was considered to be an intermediate-grade uterine smooth muscle tumor (USMT) . As of now, ALM is a clinically benign disease, characterized by an absence in its contribution to fatalities in patients [85]. Due to its unusual presentation of histologic features, these tumors have been known as: atypical leiomyoma, symplastic leiomyoma, and leiomyoma with bizarre nuclei (Fig. 6.7). The tumor origin and histogenesis of ALM remain largely unknown. Recently, several studies attempted to reexamine and evaluate the nature of ALM using the tumors’ clinical, histology, and immunohistochemistry aspects [56, 86–88]. Despite the benign clinical course of ALM, the overlap of several histological features and immunoprofiles were found between ALM and LMS. The histogenesis and its relationship to LMS draw great attention. To address this issue, comparison of the molecular and gene expression patterns in these two types of tumors was investigated recently. It was found that ALM shared or was closely related to LMS in many gene mutations including P53, MED12, and PTEN [46, 60–66, 72–77, 86, 89–101] (Fig. 6.8). The findings of shared genetic and molecular alterations as well as the histologic features between ALM and LMS suggest a close relation for the histogenesis of these two different diseases (Fig. 6.9) [86]. In fact, the early changes of ALM and tumor progression of LMS may be far more complex than we expected, and the findings of the shared genetic changes between ALM and LMS may either truly reflect the stepwise tumor progression or require some as of yet unidentified molecular changes which occur only in LMS but not ALM. Additional studies are needed to further characterize the nature of ALM.
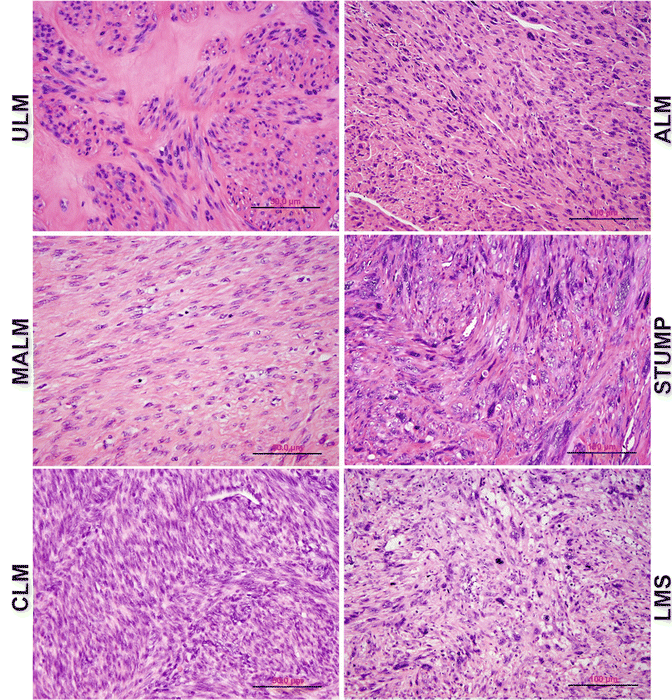
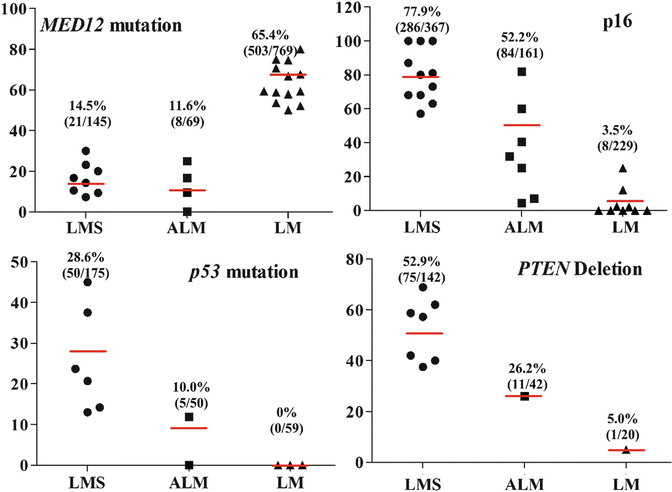

Fig. 6.7
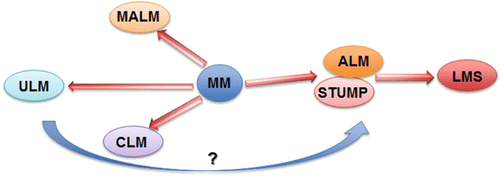
Histologic variants of uterine smooth muscle tumors (USMTs) . Photomicrographs illustrate the histologic examples of six USMT variants, including usual type leiomyoma (ULM), cellular leiomyomas (CLM), mitotically active leiomyomas (MALM), atypical leiomyomas (ALMs), uterine smooth muscle tumors of uncertain malignant potential (STUMP), and leiomyosarcomas (LMSs)

Fig. 6.8
Dot plot analyses summarized available published data for the gene mutations and expression pattern among leiomyoma (LM, triangles), atypical leiomyoma (ALM, squares), and leiomyosarcoma (LMS, round) [40, 43, 45, 46, 49–56, 60–66, 72–77, 86, 89–101]. The average rates of mutations for each category are listed above
Uterine smooth muscle tumors of uncertain malignant potential (STUMP) show unequivocal histological features (i.e., coagulative tumor cell necrosis, nuclear atypia, and mitotic activity), but do not meet all diagnostic criteria for LMS, ULM, or its variants [3]. STUMP is a heterogeneous group tumors which cannot be classified as definitively benign or malignant; the nature of histopathology may be benign, intermediate, or malignant (Table 6.4) [38, 84]. Therefore, STUMP is an entity for diagnosis of exclusion. The mean age at diagnosis was 45 years, which is younger than LMS and similar to leiomyoma variants [5, 86]. The incidence of STUMP is also unknown. The recurrence rate in patients with uterine STUMP was 6.7 % in comparison to LMS at 66.7 % recurrence [102–104]. Consequently, STUMP is considered also as an intermediate or early malignant tumor type [86, 102–104]. The relationship between STUMP and LMS has not yet been fully established.
Standard | |
|---|---|
ALM | Cytological atypia: in 4×, focal, multifocal, or diffuse moderate to severe cytological atypia |
Mitosis figure: <5 MF/10 HPF | |
Necrosis: Absent | |
STUMP | Tumor cell necrosis in a typical leiomyoma |
Necrosis of uncertain type with ≥10 MF/10 HPFs or marked diffuse atypia | |
Marked diffuse or focal atypia with borderline mitotic counts | |
Necrosis difficult to classify Problematic finding such as epithelioid or myxoid change is present |
Differential diagnosis of STUMP, ALM, and LMS can be challenging, and several markers such as P53, P16, FASCIN, Ki-67, ER, and PR can be potentially used for diagnosis. For example, P53 mutations are slightly lower in STUMP [23 % (7/30)] and ALM [18 % (12/112)] than in LMS (20–43 %) [46, 61–66] (Fig. 6.8). P16 is an important marker for LMS where diffuse immunoreactivity for P16 is present in 79 % (260/329) of LMS, 56 % (67/119) of STUMP, and 32 % (13/41) of ALM, respectively (Fig. 6.8) [40, 43, 45, 49–56]. FASCIN expression is also similar between STUMP [50 % (2/4)] and LMS [79 % (31/39)] [57, 58].
Recent identification of MED12 mutations in ULMs can be a useful marker in the differential diagnosis. Nearly 70 % of ULMs harbor MED12 mutations, but the mutation rate is very low in STUMP, ALM, and ULM (less than 15 %, Fig. 6.8) [60, 86, 89–101].
Based on the pattern of the molecular alterations, some STUMP may represent early or precursor lesions of LMS (Fig. 6.9).
Endometrial Stromal Sarcomas
Clinical Features
Endo metrial stromal sarcoma (ESS) accounts for approximately ~10–15 % of all uterine sarcomas and occurs over a wide age range with a mean age of 50 years old [35, 105]. Patients commonly present with abnormal uterine bleeding, pelvic pain, pelvic mass, and dysmenorrhea but as many as 25 % of them are asymptomatic [106]. Occasionally, metastasis may be the initial presentation. The cause of the disease is unknown but may be related to obesity, diabetes, ovarian polycystic disease, estrogen, tamoxifen therapy, and pelvic radiation [107, 108]. Most cases (60 %) present with FIGO stage I disease, and the remainder are in stages II–IV [105].
Pathological Features
According to 2014 WHO classification, endometrial stromal tumors (ESTs) can be divided into three subtypes: endometrial stromal nodule (ESN), low-grade (LGESS)/high-grade (HGESS) endometrial stromal sarcoma, and undifferentiated uterine sarcoma (UUS) on the basis of cytologic atypia, differentiation, mitotic count, and immunoprofile (Fig. 6.10) [3]. ESN is a benign endometrial stromal tumor that has a well-circumscribed margin. For ESS, the tumor size ranges from 5 to 10 cm, and the cut surface is usually yellow to tan with areas of hemorrhage and possible necrosis [105, 106]. Tumors grow polypoid or intramural masses. The common growth pattern is wormlike plugs of tumor that fill and distend to the myometrial veins (intravascular) with frequent extension to parametrical veins and lymphatics. LGESS shows minimal to no cytological atypia and low mitotic activity (usually <5/10 HPF). The mitotic activity of HGESS is typically >10/10 HPF and is typically very striking [109].
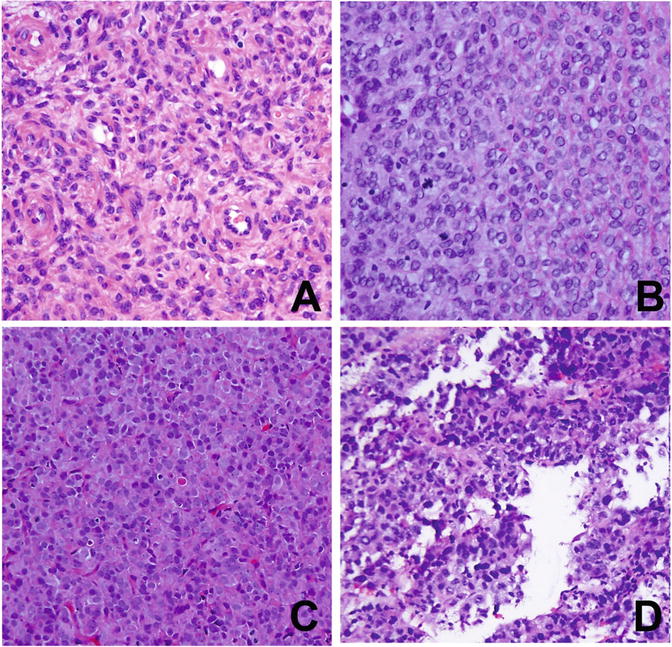

Fig. 6.10
Histology and cytology of uterine stromal sarcoma. (a) Low-grade endometrial stromal sarcoma (LGESS). (b) High-grade endometrial stromal sarcoma (HGESS). (c) Uterine undifferentiated sarcoma of uniform type (USS). (d) Uterine undifferentiated sarcoma of pleomorphic type
Immunohistochemistry and Genetic Aberrations
CD10, a membrane glycoprotein that functions as a cell surface enzyme, is a feature marker for ESS and is diffusely positive in almost all ESS. ER (only α-isoform), PR, vimentin, α-SMA, and keratin are also immunoreactive in ESS [110–112]. Nuclear β-catenin and WT-1 can be positive in ESS [113–115], whereas desmin, h-caldesmin, and HDCA8 are generally negative [112, 116]. C-Kit can be positive, but C-Kit mutations are not observed [117]. In those ESSs with smooth muscle differentiation or sex cord-like differentiation, the heterologous elements are usually reactive for smooth muscle markers, CD10, inhibin, calretinin, melan-A, CD99, and WT-1 [112, 118]. Interferon-induced transmembrane protein 1 (IFITM1) recently has been found as a sensitive and specific marker for endometrial stromal differentiation across the spectrum from proliferative endometrium to metastatic stromal sarcoma. IFITM1 has been reported to be highly sensitive and specific in the distinction between endometrial stromal tumors and uterine smooth muscle tumors (72.7 % and 86.7 %, respectively) [119].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree