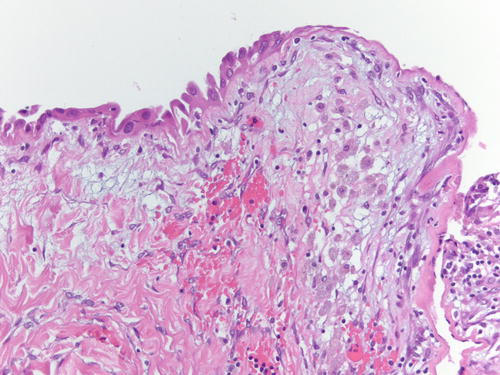
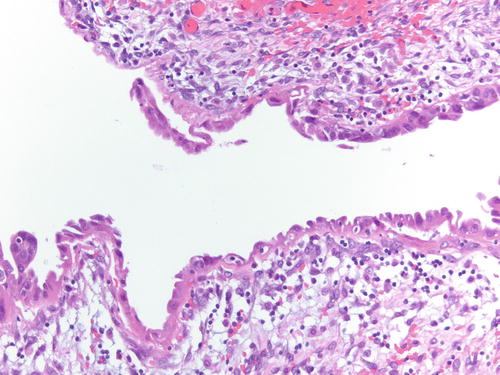
Fig. 3.1
Low power examination of ovarian endometriotic cyst lined by epithelium and underlying stroma with remote hemorrhage represented by pigment-laden macrophages. H&E 20×
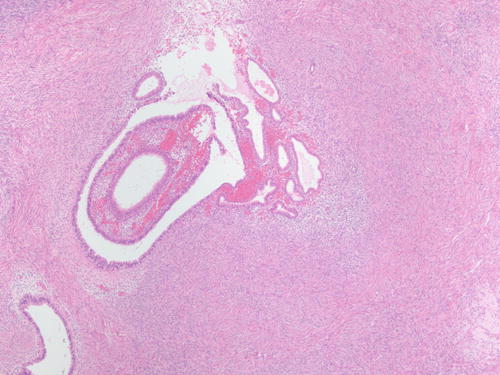
Fig. 3.2
Ovarian stroma with endometrial-type glands associated with endometrial-type stroma and recent hemorrhage. H&E 400×
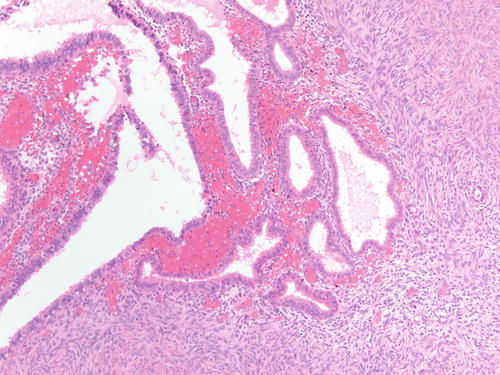
Fig. 3.3
High-power examination of Fig. 3.2 to better demonstrate endometrial-type glands and stroma devoid of atypia. H&E 200×
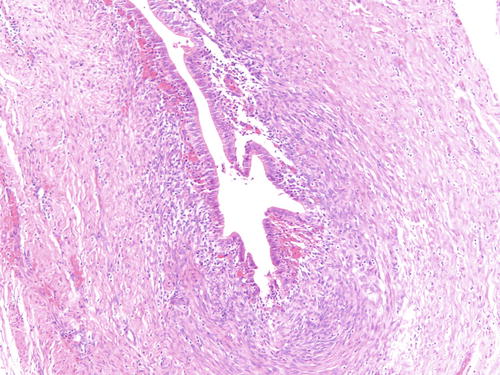
Fig. 3.4
Example of endometriosis better demonstrating cellular endometrial-type stroma. H&E 200×
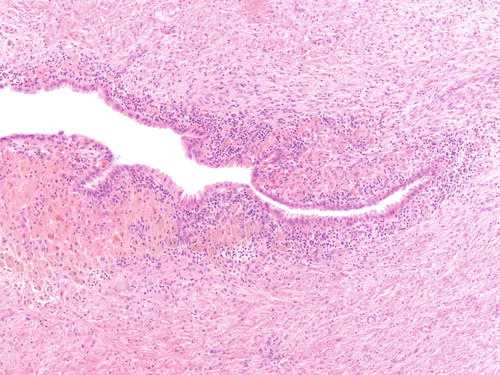
Fig. 3.5
Ovarian endometriotic cyst with abundant pigment-laden macrophages. H&E 200×
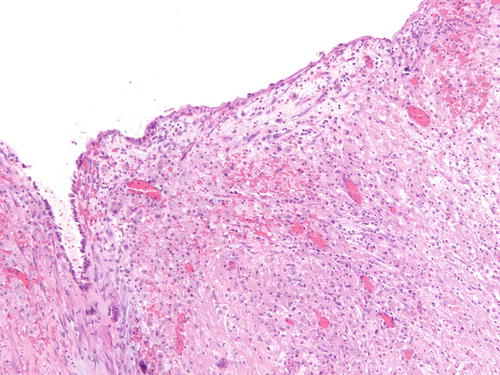
Fig. 3.6
Adjacent area to Fig. 3.5 that is a more challenging example of endometriosis with abundant histiocytes. H&E 200×
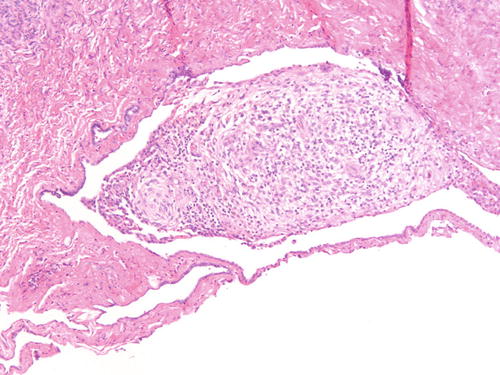
Fig. 3.7
Ovarian surface adhesions with clusters of stromal only cells with decidualized changes in patient with known endometriosis receiving hormonal treatment. H&E 200×
While the endometrial-type cysts or glands in endometriosis have an epithelium that resembles the endometrial epithelium, the same metaplastic changes that occur in the endometrium can be seen in endometriotic lesions obscuring or making the diagnosis more challenging (Fig. 3.8). Similarly, the stroma might be overlooked or misinterpreted as cellular ovarian stroma and may be very subtle and or barely perceptible and discontinuous to the periglandular zone, while many other lesions can demonstrate areas of hemorrhage (Figs. 3.9, 3.10, and 3.11).
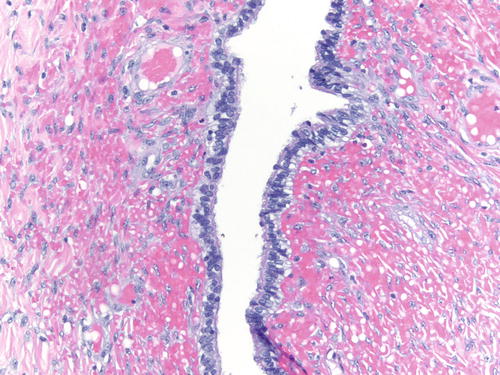
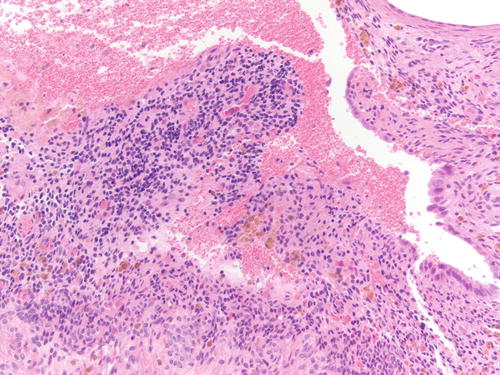
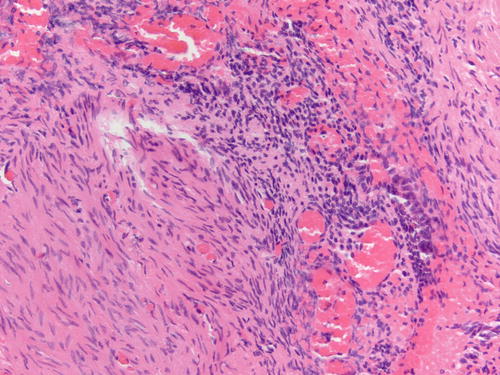
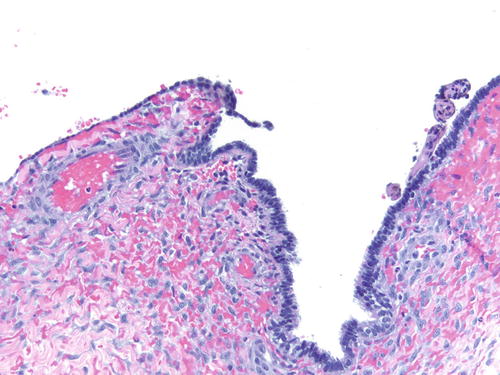

Fig. 3.8
Area of endometriosis with ciliated metaplasia as well as clear cell change on the right, in an otherwise classic example of endometriosis . H&E 200×

Fig. 3.9
Stroma predominant endometriosis with focal endometrial-type epithelium on the right. Also note pigment-laden macrophages consistent with remote hemorrhage. H&E 200×

Fig. 3.10
Example of stroma predominant endometriosis with focal endometrial-type epithelium on the right. Note recent hemorrhagic foci. H&E 200×

Fig. 3.11
Same case as Fig. 3.8 showing predominantly ciliated epithelium, not supportive of endometriosis. Also note lack of endometrial-type stroma. Other areas had classic features of endometriosis . H&E 200×
History of Endometriosis
Most of our knowledge of endometriosis can be traced back to Dr. John Albertson Sampson’s publications. However, the first description of ovarian endometriosis is credited to William Wood Russell, who presented a paper to the Johns Hopkins Medical Society in 1899 entitled “Aberrant portions of the Müllerian duct found in the ovary” [7]. Pick later reported that Rokitansky’s cystosarcoma adenoides ovarii uterinum, described by the latter in his textbook of pathologic anatomy published in 1861, could represent ovarian endometriosis [7].
Endometriosis was the primary focus of research of Dr. Sampson from 1921 and continued until the end of his career [7–9]. These contributions earned him the appellation “Father of Endometriosis.” Not only were these articles of significant scientific importance, but they were also written solely by him, but their number (a total of 18, 14 published in the 1920s), their length (mean 35 pages), and their numerous gross and microscopic illustrations would be distinctly unusual today. Most of the work was conducted by himself in his own laboratory and with his own technician, independent of the Pathology Department of Albany Hospital, where he worked [7]. In 1936, Cattell and Swinton reviewed the literature prior to 1921, estimating that fewer than 20 reports of what would be interpreted today as endometriosis were published worldwide to that date [7].
The first report of extraovarian endometriosis is credited to Rokitansky in 1860, referring extraovarian endometriotic lesions as “adenomyomas” because of the frequent admixture of endometrial tissue with benign smooth muscle. That report was followed by similar reports by von Recklinghausen between 1893 and 1896. Originally, endometriosis was considered a congenital lesion of either Wolffian or Müllerian origin. This theory was subsequently supplanted by the coelomic or serosal metaplasia theory (attributed to Iwanoff). In his 1898 article, Iwanoff proposed that ectopic endometrial tissue is a result of metaplasia of the peritoneum. Robert Meyer believed that peritoneal inflammation stimulated the metaplastic transformation of the mesothelium to endometrial-like tissue [7]. A more detailed description of Sampson’s papers and the history of endometriosis can be found in the excellent publications by Dr. Philip Clement [7–9].
Origin of Endometriosis
Much on this topic has been published and debated, but it is still controversial. In Sampson’s first paper, he postulated the idea that endometriosis could be due to two possibilities. The endometrium during normal menstrual period might take a backward direction and flow into the peritoneal cavity and attach to any organ, a concept that later would develop into the implantation theory (abnormal menstruation with a backward flow through the tube). Sampson postulated that “menstrual blood might at times escape into the pelvis, carrying with it some of the epithelium lining the cyst cavity; this epithelium may become implanted in the cul-de-sac or other portions of the pelvis and there give rise to other foci of endometrial tissue” (Fig. 3.12). Another possibility was that an ovarian endometrioma may rupture, spreading its endometrial-type lining into the peritoneum, indicating that at the time, endometrioma or ovarian “hematoma ” was believed as an independent lesion and not associating that ovarian endometriosis might also be related to transtubal spread of endometrial tissue [7, 10, 11]. Evidence for the theory proposed by Sampson included finding the endometriotic lesions during the menstrual life of women, the presence of a retroflexed uterus favoring a backward flow of menstrual blood, patent tubes indicating that transtubal spread was possible, and the occurrence of endometriosis in the most susceptible areas of the ovary for this occurrence [7] (Fig. 3.13). Additional observations that supported his theory were reported in subsequent publications including “…blood may be observed escaping through the lumen of the fimbriated ends of the tubes of women operated upon during menstruation…” and finding endometrial tissue within tubal lumina in removed fallopian tubes [7] (Figs. 3.14 and 3.15). In 1926, he w as able to quote in support of this belief the experimental work of Jacobson who successfully “transplanted” endometrial tissue into the peritoneum of rabbits [12]. In a paper published in 1927, Sampson reported a case of embolic endometriosis in the venous circulation . He described a patient with myomatous uteri who was menstruating at the time of surgery. Injecting the uterine cavity with water and melted gelating, he noted how the menstruating blood was “escaping from the severed uterine and ovarian veins” [13, 14]. He suggested that “bits of uterine mucosa, occasionally, might escape into the venous circulation during menstruation” adding anot her possibility to the pathways of endometriosis. It was also Sampson who introduced the term endometriosis: “The nomenclature of misplaced endometrial or Müllerian lesions is a difficult one to decide upon….A variety of lesions is produced by misplaced endometrial or Müllerian tissue, and it is difficult to classify all of them as true tumors….The term endometriosis is more descriptive than mullerianosis and is correct in the majority of instances because we believe that the uterine mucosa is the chief source of these lesions” [7].
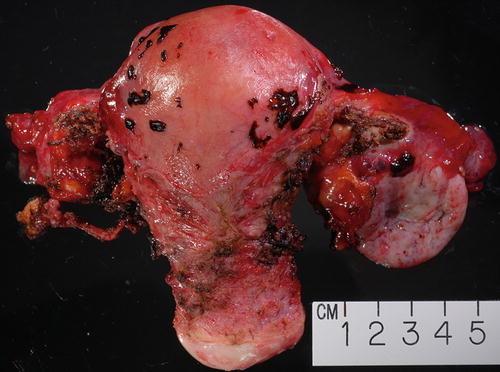
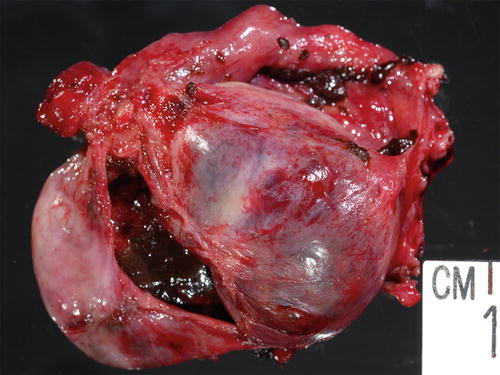
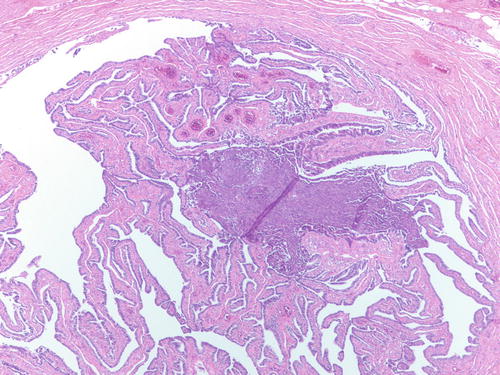
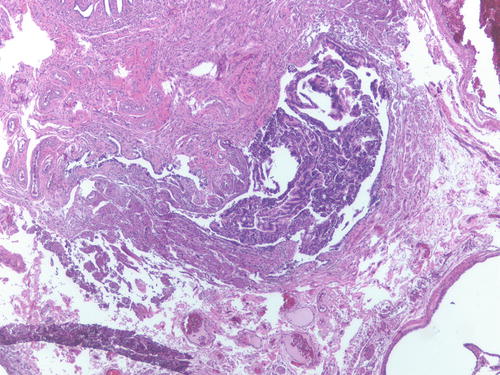

Fig. 3.12
Gross example of endometriosis involving uterine serosa as well as ovarian surface on the right. The enlarged ovary was in part replaced by endometriotic cyst

Fig. 3.13
Enlarged ovary replaced by cystic lesion containing chocolate-type content grossly consistent with endometriosis

Fig. 3.14
Endometrial-type tissue involving the lumen of an otherwise normal fallopian tube, suggesting that endometrial tissue can travel through the tube. H&E 100×

Fig. 3.15
Patient had multiple foci of endometriosis involving ovaries, uterine serosa, and peritoneum as well as endometrial-type tissue involving the fallopian tube lumen. H&E 100×
However, Sampson’s work provoked a revival of the metaplasia theory proposed by Meyer [12]. Criticisms on the retrograde menstruation were based on the belief that sloughed endometrial tissue had become anoxic and nonviabl e and accordingly was unable to grow at another location [10, 13]. Novak, reexamining the histology of hundreds of fallopian tubes, found particles of uterine tissue lying free in the lumen of the tube in only seven instances. In none had the patient been menstruating. In at least five of them, the endometrial tissue was so large that it was not possible for them to have entered through the tiny tubes orifice, and as endometrial tissue was also found in a number of the adjacent ovaries, Novak believed that the fragments were traveling down the tube and not up [13, 15]. He ch allenged the retrograde menstrual theory to explain such a common condition as pelvic endometriosis. The proponents of the metaplasia theory pointed out that all the genital epithelia were derived from the coelomic epithelium and so are related to the peritoneum, indicating that an irritant can induce the peritoneum to transform itself into endometrial tissue, since the endometrium and endosalpinx may be looked on as modified peritoneum [13, 15].
There is still debate today about the origin of endometriosis, and it is possible that there is more than one cause of endometriosis, with the dominant causative factors varying depending on the location. There are three clinically distinct forms of endometriosis, including endometriotic implants on the surface of the pelvic peritoneum and ovaries (peritoneal endometriosis), ovarian cysts lined by endometrioid mucosa (endometriomas), and complex solid masses comprised of endometriotic tissue within adipose and fibromuscular tissue, residing between the rectum and the vagina (rectovaginal endometriotic nodule) [16]. Their causes could be the same or different in each site. What is known since the early twentieth century is that endometriosis is related to uninterrupted ovulatory cycles [16]. It was felt that the increase in the endometriotic rate in the 1930s was due to delayed marriages, the lack of early child bearing, probably associated with the economic difficulties of the great depression era [15]. Meigs agreed with these concepts and recommended young couples to have children early and practice contraception after (not before) they have children: “couples should be taught how to have children, not to avoid them” [15].
Based on the lack of uniform opinion (and definitive research models proving or disproving these theories) regarding the origin of endometriosis in the ovary (or others sites), different models are currently being proposed. Proponents of the coelomic metaplasia theory support that the peritoneal–mesothelial covering of the adult ovary retains the ability to differentiate into serous, endometrioid, and mucinous epithelia, acquiring a Müllerian epithelium [16, 17]. Recently a different theory has been proposed: direct implantation of tubal epithelium into the ovary to form an inclusion cyst, which in turn might be the site of origin of ovarian serous carcinoma is an alternative theory to that of metaplasia from the surface epithelium (mesothelium) [4] (Figs. 3.16 and 3.17). Implantation of fallopian tube epithelium from the fimbria at the time of ovulation when the surface epithelium is disrupted can explain the derivation of low-grade and high-grade serous carcinomas [4]. Also, entrapment of exfoliated endometrial epithelium, most likely in areas of prior ovulation can be incorporated into the ovarian tissue, developing into endometriotic cysts [4, 5].
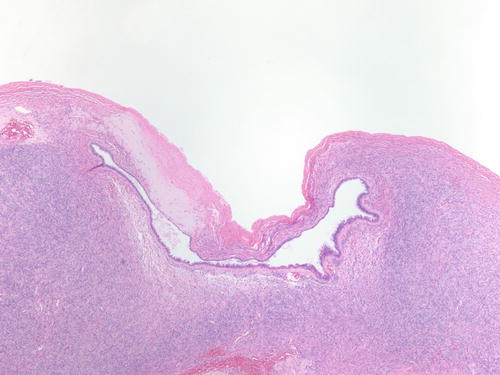
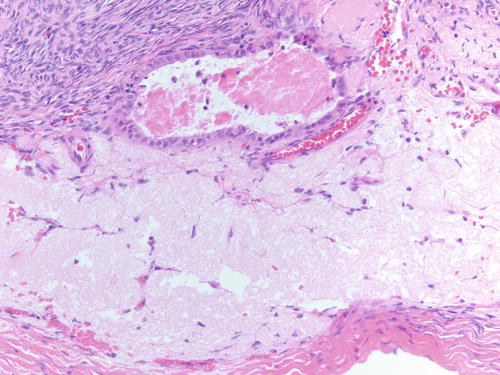

Fig. 3.16
Epithelial-lined cyst adjacent to ovarian surface with area of hyalinized stroma . Possible are implantations of fallopian tube epithelium after ovulation. H&E 40×

Fig. 3.17
High-power examination of epithelial-lined cyst adjacent to ovarian surface with area of hyalinized stroma. H&E 200×
Irrespective of the cause, it is unclear what determines the fact that some, but not most, women develops endometriosis, since most women have backflow menstruation into the peritoneal cavity, but endometriosis occurs in only 5–10 % [18]. Two mechanisms could potentially explain the preferential implantation of endometrial tissue onto the peritoneal surface in some patients: molecular defects (activation of oncogenic pathways) and/or immunologic abnormalities (failure of the immune system to clear implants from the peritoneal surface) [18–26].
Metaplastic Changes and Atypical Morphologic Features of Endometriosis
Before molecular and/or genetic studies were widely available, morphologic features were identified to try to predict or detect patients with endometriosis at risk of developing malignancy. The first lesions described were atypical endometriosis and hyperplasia within an ovarian endometriotic cyst [27–44]. In some cases, both terms were used interchangeably; more specifically, cases of hyperplasia within ovarian endometriosis were designated atypical endometriosis, similar to cases of ovarian endometriosis harboring only cytologic atypia. Atypical endometriosis is usually characterized by a focal or multifocal finding of an eosinophilic epithelium, though in some cases, focal clear cytoplasm could be seen, in addition to cells with irregular nuclei of variable size, enlarged or with bizarre changes, and/or hyperchromatic with smudged chromatin and/or prominent nucleoli (Figs. 3.18, 3.19, 3.20, 3.21, and 3.22). The atypical cells are lined in a simple epithelium, but the cysts may be focally stratified as small papillary structures [45]. Inflammatory cells can accompany this epithelium. It is often difficult to determine the true nature of these changes, which may range from being entirely reactive changes (and accordingly benign) to, at the opposite end of the spectrum, being compatible with a neoplasm and mimicking or suggesting a clear cell carcinoma (Figs. 3.22, 3.23, 3.24, and 3.25). Frequently the epithelium is discontinuous, leaving the underlying stroma with hemosiderin-laden macrophages, pseudoxanthoma cells, fibrinous material, and/or loose fibrous tissue with hemorrhage. Epithelial stratification and/or papillae can be seen [27]. Cases of hyperplasia within endometrioma show similar features to that seen in the endometrium, including crowded, simple, or complex glands with or without cytologic atypia (Figs. 3.26, 3.27, 3.28, and 3.29).
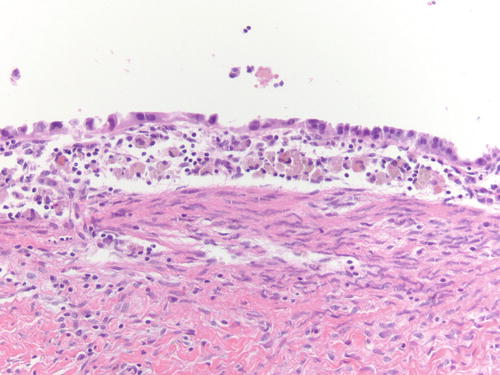
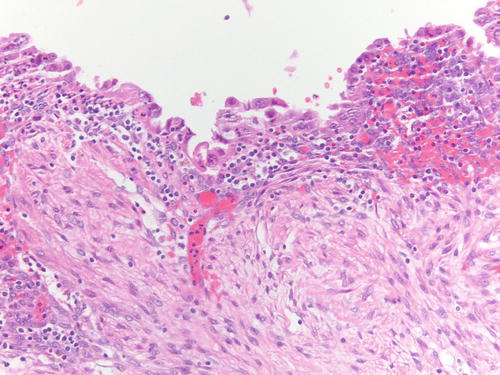
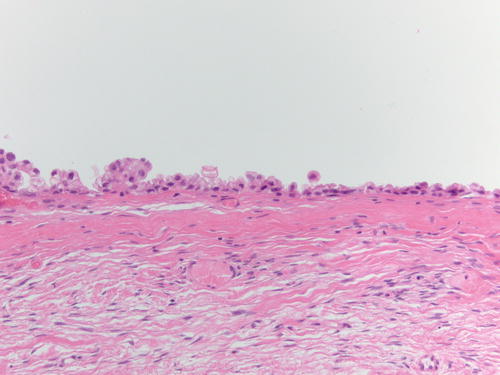
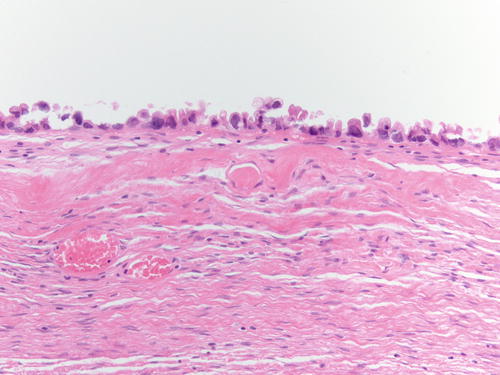
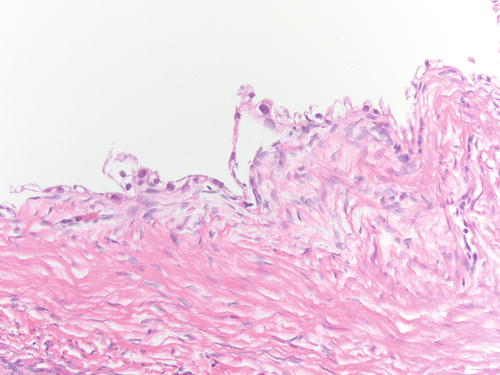
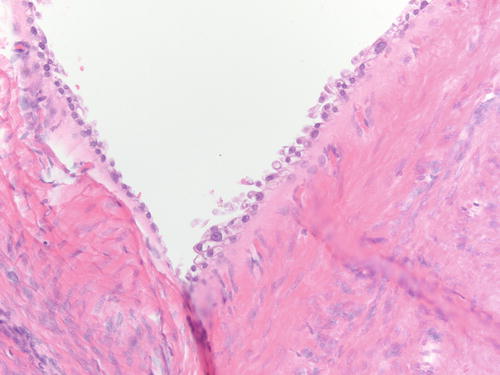
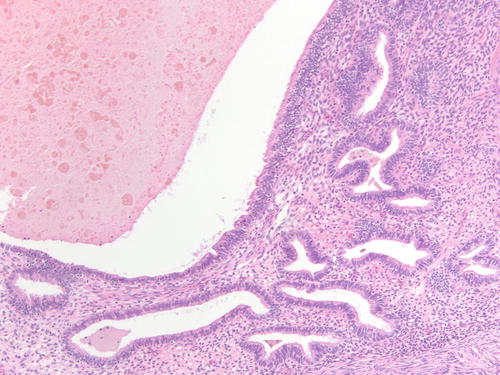
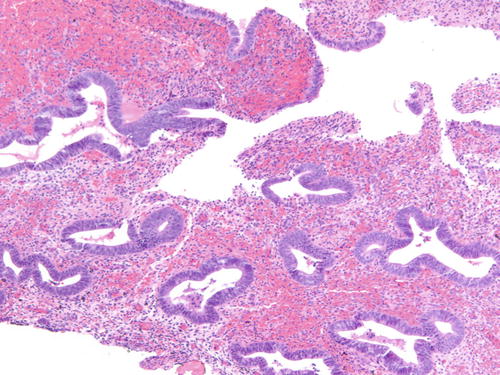
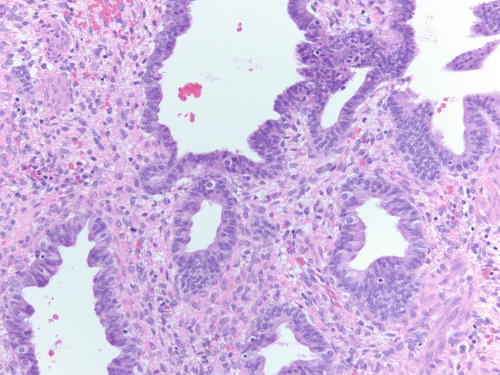
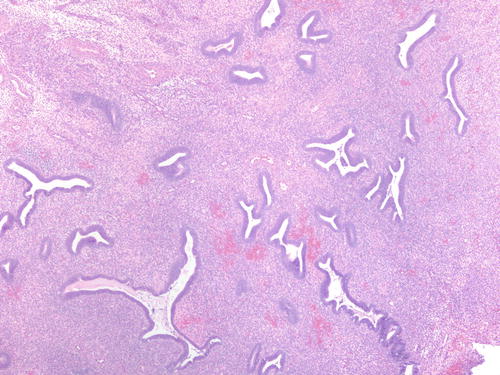

Fig. 3.18
Area of endometriotic cyst showing epithelial lining with larger nuclei, pleomorphism, and high nuclear to cytoplasmic ratio, consistent with atypical endometriosis. H&E 200×

Fig. 3.19
Another example of atypical endometriosis. In this case, there is abundant inflammation, and the changes could be reactive in nature. H&E 200×

Fig. 3.22
Atypical endometriosis. In this case, the cytoplasm shows clear cell change and incipient papillary formations, raising the possibility of progression to clear cell carcinoma. H&E 100×

Fig. 3.23
High-power examination showing clear cell change in the cytoplasm. Other areas showed classic solid and glandular features of clear cell carcinoma. H&E 200×

Fig. 3.24
Examples of atypical clear cells lining an endometriotic cyst suggesting incipient clear cell carcinoma (or carcinoma in situ). H&E 200×

Fig. 3.25
Examples of atypical clear cells lining an endometriotic cyst suggesting incipient clear cell carcinoma (or carcinoma in situ). H&E 200×

Fig. 3.26
Clustered endometrial glands in endometriotic cyst, consistent with hyperplasia within ovarian endometriosis. H&E 200×

Fig. 3.27
Detached fragments of hyperplastic endometrial glands within a large endometriotic cyst. H&E 100×

Fig. 3.28
High-power examination showing cytologic atypia consisting of focal enlarged round nuclei and prominent nucleoli. H&E 200×

Fig. 3.29
Low-power examination of polypoid area within an endometriotic cyst, consistent with polypoid endometriosis, differential diagnosis of hyperplasia. H&E 40×
The rate of atypical endometriosis is very low, in the range of 1.7–3.6 % as reported by Fukunaga et al. and Czernobilsky and Morris [28, 29]. The rate of malignant transformation in atypical endometriosis was 25 % in Fukunaga’s study (one of four patients with atypical endometriosis developed carcinoma after 2.5 years of follow-up). While in Seidman’s study, only 1 of 20 patients with complex atypical hyperplasia or “early carcinoma” developed clinically evident endometrioid adenocarcinoma after 8.6 years of follow-up [28, 33]. A recent report described the case of a patient that started with ovarian endometriosis and progressed to atypical ovarian endometriosis and ovarian endometrioid carcinoma 10 years after the original diagnosis [46].
Two things are worth emphasizing at this point: first, the importance of thorough histologic sampling of the tissue when significant atypia or hyperplasia is identified in endometriosis (submitting the entire lesion is recommended) and second, the metaplastic changes that can occur in endometriosis and mimic neoplastic changes.
Similarly to the uterine endometrium , the epithelial lining of ovarian endometriosis can undergo metaplastic, hyperplastic, and atypical changes and even malignant transformation [29, 32, 44]. Metaplasia is a replacement of the endometrial epithelium with an epithelium that is normally encountered in another organ of derivation [32, 44]. Metaplastic changes can create difficulty diagnosing the underlying endometriosis or suggesting a different diagnosis (Figs. 3.30 and 3.31). Most common changes include ciliated change, exemplifying the close relationship of tubal-type epithelium, eosinophilic, hobnail or clear cell, squamous, and mucinous metaplasia. In a study of 388 ovarian endometriosis cases, the most common type of metaplasia was eosinophilic or oncocytic (5.6 %) followed by mucinous metaplasia (5.3 %), while the other types of metaplasia accounted for less than 2 % each [32]. The majority of metaplasias in ovarian endometriosis are observed in cases not associated with malignant epithelial tumor or atypia and should not be interpreted as neoplastic features [44]. However, cases of mucinous borderline tumors, endocervical type, also associated with endometriosis usually harbor areas of endometriosis with mucinous metaplasia adjacent to the tumor [44].
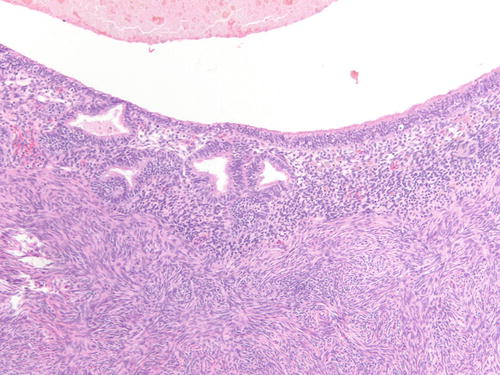
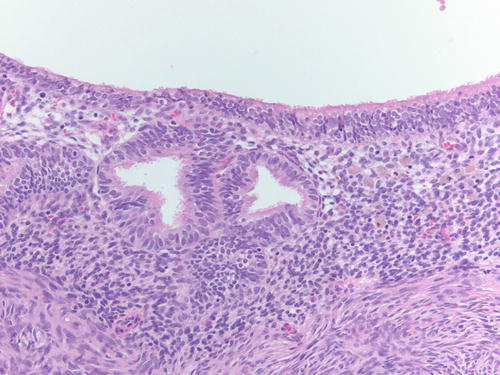

Fig. 3.30
Low- and high-power examinations of an endometriotic cyst. Note the lining with cilia, indicative of tubal metaplasia and the cellular endometrial-type stroma and occasional pigment-laden macrophages. H&E 100×

Fig. 3.31
Low- and high-power examinations of an endometriotic cyst. Note the lining with cilia, indicative of tubal metaplasia and the cellular endometrial-type stroma and occasional pigment-laden macrophages. H&E 200×
Occasionally, endometriotic glands in women with an intrauterine or ectopic pregnancy exhibit overt secretory changes that may include Arias-Stella reaction [47] (Figs. 3.32, 3.33, 3.34, and 3.35). The changes include nuclear enlargement and hyperchromasia and/or optically clear nuclei with prominent nucleoli and cytoplasmic vacuolization or clearing due to accumulation of intracellular glycogen. Arias-Stella changes in glands containing nuclei showing markedly pleomorphic features (monstrous cell type) can in occasion be seen. These changes mimic atypical endometriosis or clear cell carcinoma. The history of pregnancy, or changes seen in a young and fertile patient, might alert to the possibility of the presence of benign features. If the lesion presents in postmenopausal patients or if the differential diagnosis is still problematic, immunostains for KI-67 and Estrogen receptors (ER) can distinguish Arias-Stella reaction from clear cell carcinoma and other types of high-grade carcinomas [48]. In one study that comparatively assessed the expression of these markers in uterine carcinomas and uterine Arias-Stella reaction [48], the former had a low KI-67 proliferation index, while clear cell carcinoma expressed KI-67 broadly with increased intensity (Fig. 3.31). ER was negative in all tested clear cell carcinoma cases, while Arias-Stella reaction was positive in about 50 % of the cases.
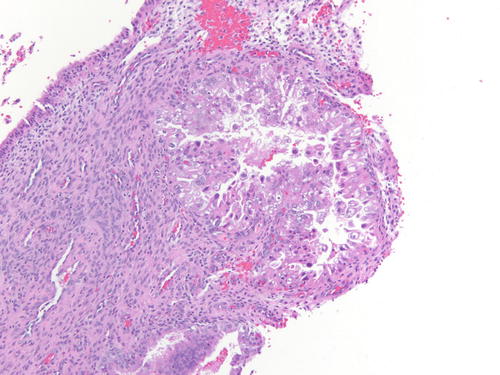
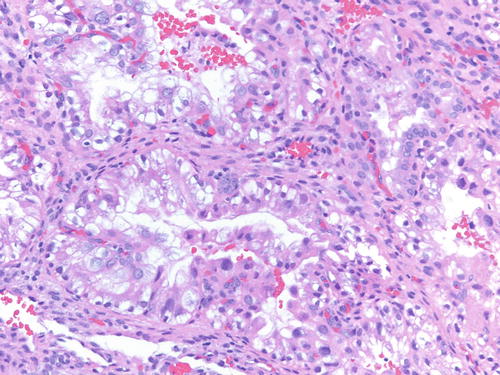
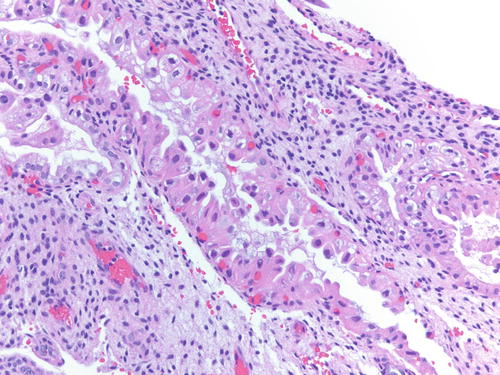
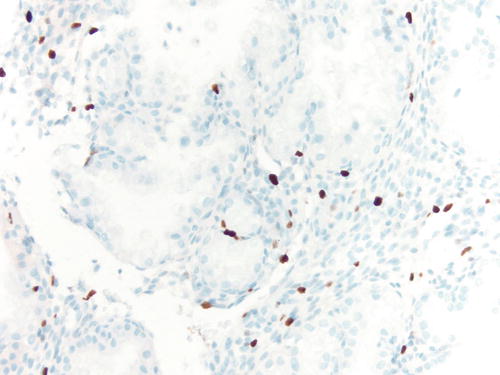

Fig. 3.32
Low power examination of Arias-Stella change within endometriotic cyst raising the possibility of clear cell carcinoma. Patient was 37 weeks pregnant, and the ovarian cyst was resected during cesarean section. H&E 100×

Fig. 3.33
High power examination of Arias-Stella change within endometriotic cyst raising the possibility of clear cell carcinoma. Patient was 37 weeks pregnant, and the ovarian cyst was resected during cesarean section. H&E 200×

Fig. 3.34
Arias-Stella change within endometriotic cyst raising the possibility of clear cell carcinoma. Patient was 37 weeks pregnant, and the ovarian cyst was resected during cesarean section. H&E 200×

Fig. 3.35
Immunostain for KI-67 in case of Arias-Stella change showing only focally positive nuclei, while clear cell carcinoma demonstrates a more diffuse staining pattern. 200×
Molecular Features of Ovarian Endometriosis and Associated Carcinomas
Molecular distinctions between endometrium and ovarian endometriosis have been detected including increase production of estrogen, prostaglandins, metalloproteinases, inflammatory cytokines, and chemokines in endometriotic tissues [20–24, 49–54]. Increased levels of acute inflammatory cytokines including several interleukins were proposed to help with implantation of endometrial tissue fragments onto ovarian and peritoneal surfaces. In addition, hormones, such as estrogen , play an important role in endometriosis, in part regulated by promoter methylation of the estrogen receptor β gene [18, 55].
Ovarian endometriosis bears genetic instability or damages caused by iron-dependent oxidative stress. DNA damage and loss of heterozygosity (LOH ) caused by oxidative stress are critical factors in the carcinogenic process with downregulation of some tumor suppressor genes and overexpression of specific candidate oncogenes implicated in tumorigenesis [56]. Different molecular changes are responsible in development of ovarian clear cell carcinoma and endometrioid carcinoma from endometriosis.
In 1996, British investigators examined for the first time DNA from endometriosis to analyze if these samples harbored alterations that could also be seen in ovarian endometrioid carcinoma [57]. While the cases of endometriosis did not harbor alterations in TP53 and KRAS genes, allelic losses in tumor suppressor genes located on chromosome 9p (18 %), 11q (18 %), and 22q (15 %) were identified. Eleven (28 %) of the 40 cases demonstrated LOH at one or more of these loci. In a latter study, Sato et al. evaluated the tumor suppressor gene PTEN/MMAC1, located on chromosome arm 10q (10q23.3) [58]. LOH at this site occurred in 56 % of endometriotic cysts, similar to that seen in 42 % ovarian endometrioid carcinomas and 27 % clear cell carcinomas. Somatic mutations in phosphatase and tensin homolog (PTEN) were identified in 20 % ovarian endometrioid carcinomas, 8 % ovarian clear cell carcinomas, and 20 % endometriotic cysts.
Other studies have found LOH within ovarian endometriosis (including atypical endometriosis) at candidate ovarian tumor suppressor gene loci and LOH events or other genetic alterations common to the endometriosis and synchronous ovarian carcinomas, including TP53 [41, 42, 59]. Recent whole genome or targeted sequencing studies have identified frequent mutations of PTEN (14–20 %), CTNNB1 (16–53.3 %), and KRAS most commonly associated with ovarian endometrioid cancer [60–63].
Based on the different morphology, it is understandable that clear cell carcinoma harbors different molecular changes, although a few commonalities exist. Kuo KT et al. demonstrated ovarian clear cell carcinomas with mutations of PIK3CA (33 %), TP53 (15 %), KRAS (7 %), PTEN (5 %), CTNNB1 (3 %), and BRAF (1 %) genes [64]. Sequence analysis of PIK3CA in 28 clear cell carcinomas and clear cell carcinoma cell lines showed a mutation frequency of 46 %. Samples with PIK3CA mutations showed intense phosphorylated AKT immunoreactivity. These findings demonstrate that ovarian clear cell carcinomas have a high frequency of activating PIK3CA mutations [64].
Additional studies evaluated PIK3CA mutation in clear cell carcinomas. Yamamoto et al. detected somatic mutations of the PIK3CA gene in 10/23 (43 %) ovarian clear cell carcinomas, and in all cases the type of mutation was H1047R in the kinase domain [65]. The identical H1047R mutation was also detected in the coexisting endometriotic epithelium, adjacent to the clear cell carcinoma, in nine of ten (90 %) cases. Moreover, in six of the nine lesions, the H1047R mutation was identified even in the endometrioses lacking cytologic atypia supporting evidence of endometriosis as a precursor lesion. In a subsequent study, these investigators increased the sample size to 88 informative cases, and PIK3CA gene mutations were identified in 39 % of cases [66]. Findings also associated with the mutation included cystic tumor, the presence of adjacent endometriosis, prominent papillary architecture of tumor growth, the presence of hyalinized and mucoid stroma, and the absence of clear cell adenofibroma components [66].
Protein markers specific for ovarian clear cell carcinoma associated with endometriosis have also been evaluated. Hepatocyte nuclear factor-1β (HNF-1β ), a transcription factor shown to be significantly upregulated in ovarian clear cell carcinoma, is rarely expressed in ovarian non-clear cell carcinoma specimens [67], while endometrial non-clear cell carcinomas have varied staining [68]. Similarly, Kato et al. identified expression of HNF-1β in 9 of 17 clear cell tumors associated with endometriosis, including five cases of reactive endometriotic epithelium and four cases of atypical endometriosis [68]. In the same study, 16 of 40 cases of endometriosis not associated with a primary clear cell ovarian carcinoma also displayed HNF-1β expression . However, the expression of HNF-1β was almost exclusively detected in the epithelium showing inflammatory atypia [69].
Lastly, molecular/genetic changes have been identified in endometriotic lesions adjacent to malignancy, linking endometriosis, clear cell carcinoma, and endometrioid carcinoma. Wiegand et al. identified mutations of the tumor suppressor gene ARID1A that are common to endometrioid and clear cell ovarian carcinomas [70]. In their study, mutations in AT-rich interactive domain-containing protein 1A (ARID1A) were seen in 55 of 119 ovarian clear cell carcinomas (46 %), 10 of 33 endometrioid carcinomas (30 %), and none of the 76 high-grade serous ovarian carcinomas [70]. A total of 17 samples (12 of ovarian clear cell carcinoma and 5 of endometrioid carcinoma) each had two validated ARID1A mutations. In addition, immunohistochemical expression loss of BAF250a protein correlated strongly with the ovarian clear cell carcinoma and endometrioid carcinoma subtypes and the presence of ARID1A mutations, implicating ARID1A as a tumor suppressor gene frequently disrupted in ovarian clear cell and endometrioid carcinomas. Two patients with ovarian clear cell carcinomas carrying ARID1A mutations had contiguous atypical endometriosis. Both cases demonstrated ARID1A mutations and loss expression of BAF250a protein in the clear cell carcinoma and the contiguous, atypical endometriosis, but not in distant areas of endometriosis [70]. HNF-1β was expressed in the ovarian clear cell carcinoma but not in the contiguous atypical or distant endometriosis.
In an immunohistochemical evaluation of ARID1A (BAF250a) protein expression in 90 clear cell carcinoma cases, Yamamoto et al. reported the intensity of immunoreactivity for BAF250a as negative, weakly positive, and strongly positive in 44 %, 22 %, and 33 % of tumors, respectively. Compared to tumors immunoreactive for BAF250a, BAF250a-negative tumors were significantly associated with the presence of adjacent endometriosis and more frequently harbored PIK3CA mutations (P = 0.013) [66].
The same group analyzed PIK3CA mutation and ARID1A immunoreactivity in ovarian clear cell carcinomas associated with endometriosis and clear cell adenofibroma (another precursor lesion of clear cell carcinoma described later) [71]. ARID1A immunoreactivity was deficient in 17 (61 %) of the 28 endometriosis-associated carcinomas and 6 (43 %) of the 14 adenofibroma-associated carcinomas. Among the precursor lesions adjacent to the 23 ARID1A-deficient carcinomas, 86 % of the classic endometriosis (12 of 14) and 100 % of the atypical endometriosis (14 of 14), benign (3 of 3), and borderline (6 of 6) clear cell adenofibroma components were ARID1A deficient. In contrast, in the 19 patients with ARID1A-intact carcinomas, all of the adjacent precursor lesions retained ARID1A expression regardless of their types and presence/absence of atypia. Analysis of 22 solitary endometrioses and 10 endometrioses distant from ARID1A-deficient carcinomas showed that all of these lesions diffusely expressed ARID1A. Among the 42 clear cell carcinomas, somatic mutations of PIK3CA were detected in 17 (40 %) tumors. The majority (71 %) of these were ARID1A-deficient carcinomas. These results suggest that loss of ARID1A protein expression occurs as a very early event in ovarian clear cell carcinoma development, similar to the pattern of PIK3CA mutation, and frequently coexists with PIK3CA mutations [71]. This indicates that the loss of BAF250a protein expression is suggestive for the presence of ARID1A mutations and represents a useful marker of malignant transformation of endometriosis [62].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree