Introduction
The respiratory system undergoes a number of changes during pregnancy so that the gravid woman can meet the metabolic needs of both the mother and the fetus. The implications of these changes for the diagnosis and management of specific pulmonary diseases as well as for pregnancy will be considered in this chapter.
Physiologic adapations to pregnancy
Anatomic changes
Many anatomic changes occur in and around the respiratory system. In the upper airway, hyperemia and glandular hyperactivity are observed in pregnancy and are associated with edema and friability. These changes are likely the result of the expansion of plasma volume that starts early in pregnancy and progresses with increasing gestational age. Also contributing to this is the indirect effect of the elevated levels of estrogens. Consequently, up to 30% of pregnant women suffer from nasal congestion and epistaxis. This condition is known as gestational rhinitis and it usually resolves very quickly after delivery. Other consequences of mucosal edema of the upper airway include difficulties in airway management and failed endotracheal intubations as well as problems with the introduction of nasogastric tubes, necessitating liberal lubrication and extreme care. The higher propensity to snoring in pregnancy as compared to the nonpregnant population is also related to these changes.
Changes also occur in the chest wall. The lower ribcage widens, leading to an increase in the anteroposterior and transverse diameters of the chest, resulting in an overall increase of 5–7 cm in the chest wall circumference and a widening of the costal angle by about 50%. However, impaired chest wall compliance related to the enlarging uterus can occur late in pregnancy, causing decreased total lung compliance.
Physiologic measurements in pregnancy
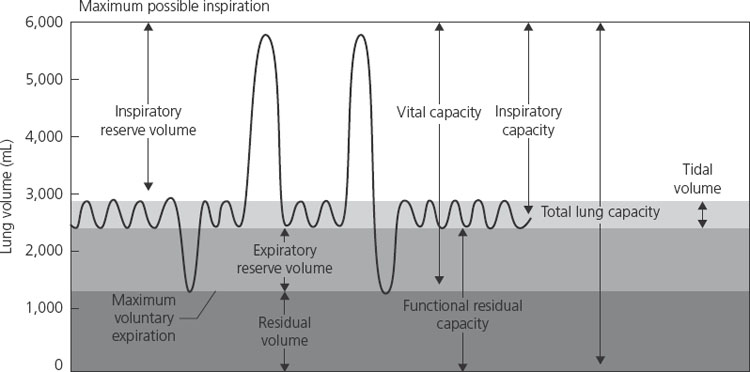
Flow rates are relatively unchanged in pregnancy. Forced expiratory volume in 1 second (FEV1), a helpful measurement in obstructive lung diseases, is not affected by pregnancy. In addition, peak expiratory flow rates are unchanged. Therefore, the interpretation and monitoring value of these tests in patients with asthma, for instance, are unchanged in pregnancy. The major effect of pregnancy on lung physiology occurs on volumes. There is an increase in tidal volume (TV) and a reduction in functional residual capacity (FRC) secondary to a decreased residual volume and expiratory reserve volume. FRC is further reduced in the supine position late in gestation. The inspiratory capacity is increased so that total lung capacity remains the same in the pregnant and nonpregnant state (Figure 1.1).
Ventilation
Minute ventilation, a product of tidal volume and respiratory rate, is increased by about 40% in pregnancy. This increase is achieved mainly by a proportional increase in tidal volume from 500 mL to 700 mL (about 40%). There is minimal change in the respiratory rate in pregnancy and any change should be interpreted as pathologic rather than physiologic.
This increase in ventilation leads to a reduction in PaCO2 levels from 35–40 mmHg in the pre-pregnant state to an average of 30 mmHg during pregnancy. The drop in PaCO2 is matched by an increased renal excretion of bicarbonate, leading to lower plasma bicarbonate levels. The end result is a plasma pH that is not significantly changed but there may be less buffering capacity in the face of an acidosis.
More profound changes occur in pregnancy at high altitude. Ventilation usually increases at high altitudes to compensate for the drop in ambient oxygen. This response in pregnancy further accentuates the drop in PaCO2 and levels of 24–28 mmHg have been reported at high altitude. Although residence at high altitude is associated with decreased maternal PaO2, intrauterine growth restriction and pre-eclampsia, modern aircraft are pressurized to about 2500 m (8200 ft) and at these pressures Huch found no evidence of ill effects on mother or fetus in 10 pregnancies studied during commercial flights [1].
The increase in ventilation and associated fall in PaCO2 occurring in pregnancy are probably due to progesterone, which may act via a number of mechanisms. Progesterone lowers the threshold and increases the sensitivity of the respiratory center to CO2. It is also possible that progesterone acts as a primary stimulant to the respiratory center independently of any change in CO2 sensitivity or threshold. Not only does progesterone stimulate ventilation, but it also increases the level of carbonic anhydrase B in the red blood cell. An increase in carbonic anhydrase will facilitate CO2 transfer, and also tends to decrease PaCO2 independently of any change in ventilation.
Oxygenation
Maternal PaO2 increases in pregnancy to 100–105 mmHg at sea level. This increase is in part secondary to an increment in cardiac output leading to an improvement in ventilation/perfusion matching in the upper lobes. The alveolar–arterial gradient (the difference between PO2 in the alveoli and that measured in the arterial blood) has been reported to increase slightly in the late stages of pregnancy from 15 to about 20. Position can also have an important effect on maternal arterial blood gases late in pregnancy. PaO2 has been shown to decrease and the alveolo-arterial gradient to increase in the supine position in pregnancy. This has been attributed to a reduction in functional residual capacity and earlier airway closing during normal tidal breathing. Alterations in cardiac output between the sitting and the supine position may also contribute to that reduction in arterial oxygen tension. Therefore, arterial blood gases are ideally obtained while pregnant women are in the sitting position.
Oxygen consumption is increased in pregnancy by about 20% and increases further during labor and delivery. About one-third of the increased oxygen consumption is necessary for the metabolism of the fetus and placenta. The remainder is supplied for the extra metabolism of the mother, in particular the extra work of increased secretion and reabsorption by the kidney.
Breathlessness in pregnancy
Approximately 50% of normal pregnant women will note dyspnea before 19 weeks gestation and 76% by 31 weeks [2]. Reasons for experiencing the sensation during normal pregnancy may be related to the effect of progesterone on the respiratory center, mechanical changes associated with weight gain or decreased venous return, and/or the demands of the fetus. Women often describe their symptoms as “needing to take a deep breath.” Since these women are otherwise normal, there should be no suggestion of cardiopulmonary disease on history or physical exam such as sudden onset of symptoms, cough, chest pain or wheezing and patients should be able to perform activities of daily living. Likewise, physical exam is normal and oxygen saturation is normal at rest and with exertion. The presence of an anemia should be sought as this is common in pregnancy. If there is no underlying disease as a cause of dyspnea, the patient can be reassured that there is no increased risk for complications during pregnancy or labor and delivery.
The remainder of this chapter discusses the most common and the most serious respiratory problems that may complicate a pregnancy. Although each requires specific management, a general approach to the care of the pulmonary patient can be found at the end of this chapter in Table 1.12.
Asthma is characterized by heightened airway responsiveness to triggers and reversible airway obstruction. According to the National Asthma Education and Prevention Program, it is defined as “a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. . .” In susceptible individuals, this inflammation causes recurrent coughing (particularly at night or early in the morning), wheezing, breathlessness, and chest tightness. These episodes are usually associated with widespread but variable airflow obstruction that is often reversible either spontaneously or with treatment [3].
Epidemiology
Asthma affects 3.7–8.4% of all pregnancies and is one of the most common serious medical complications encountered in pregnancy in the United States [4]. Other estimates from both the United Kingdom and Australia show rates close to 12–13% in pregnancy [5]. It is estimated that 10% of the population has airway hyper-responsiveness. The prevalence of asthma has significantly increased since the 1980s and cannot be solely explained by an increased awareness of the disease since the rate of death from asthma has also increased. Asthma has become the leading cause of hospitalization of children under 15 and is emerging as the most common chronic and potentially life-threatening disease of childhood, affecting one in seven children in Great Britain. Pregnant women and their children are also more likely to experience asthma than any other chronic disease.
Physiology
Asthma is a chronic inflammatory disease characterized by a reversible airway obstruction and airway hyper-reactivity. Reversible airway obstruction is defined as an obstruction on spirometry which is documented during acute attacks with normal physiology between attacks. Reversibility may also be documented by complete resolution of obstruction following the administration of a short-acting bronchodilator. Airway hyper-responsiveness, an exaggerated bronchospastic response to nonspecific agents such as methacholine and histamine or specific antigens, is the physiologic cornerstone of this disorder. Multiple factors lead to narrowing of the airway, resulting in reduced air flow, such as smooth muscle contraction, thickening of the airway wall and the presence of secretions within the airway lumen.
Pathophysiology
The predominant causes of airway obstruction in asthma include airway inflammation, cellular infiltration, and subsequent cytokine production.
Airway inflammation has many components involving cellular infiltration with Th2 lymphocytes as well as eosinophils, the former playing an important role in initiating and perpetuating inflammation through cytokine release as well as by affecting IgE production. When Th2 lymphocytes are stimulated by the appropriate antigens, they release interleukins (IL) such as IL-3, IL-4 and IL-5 as well as granulocyte macrophage-colony stimulating factor (GM-CSF). Airway cells such as smooth muscle cells and secretory cells undergo hypertrophic and hyperplastic changes whereas mast cells become sensitized with IgE and secrete tumor necrosis factor alpha (TNF-alpha), a pleiotropic inflammatory cytokine that plays a role in airway hyper-responsivess. Remodeling of the airway structure occurs as a result of collagen deposition in the basement membrane and thickening of the subepithelial connective tissue.
Clinical manifestations
Typically, patients with asthma present with periodic symptoms of shortness of breath, wheezing or chest tightness that occur in response to various stimuli. Common stimuli include exercise, cold temperatures, allergens or irritants. Common allergens could be indoor or outdoor allergens and include grass, pollen, pet dander, cockroaches, mice, dust mites and mold. A careful history should be taken regarding possible triggers and the history needs to include questioning about the home, work or even school environment.
During attacks, patients usually have expiratory wheezing which typically resolves once the symptoms improve. Depending on the severity of the attack, patients may use their accessory respiratory muscles (the parasternal, scalene, sternocleidomastoid, trapezius, and pectoralis muscles that do not normally contract with respiration) and even have paradoxic breathing (normally the abdomen should expand with inspiration but in paradoxic breathing, the abdomen may retract with inspiration, indicating marked respiratory muscle fatigue). In severe attacks leading to hyperinflation, there may be some compromise to the venous return and subsequent hypotension.
Diagnosis
The diagnosis of asthma is usually suggested by the history of episodic symptoms that follow specific triggers. The physical exam is suggestive during attacks but not when the attacks have resolved. The diagnosis is usually established by the documentation of a reversible obstruction on spirometry. Obstruction is defined on spirometry by a reduced FEV1 forced vital capacity (FVC) (normally the ratio of FEV1 to FVC should be about 75%, meaning that 75% of the total volume of a breath can be exhaled within 1 second) with variable degrees of FEV1 reduction. Normalization of FEV1 following administration of bronchodilators determines reversibility.
Spirometry is often normal in patients with asthma outside an acute attack. Establishing a diagnosis may be difficult in that case and an airway challenge may be performed to trigger an obstructive physiology. The most commonly used challenge in making the diagnosis of asthma is performed with methacholine chloride but other challenges can be used such as cold air and allergen challenges. Methacholine is a quaternary ammonium compound and likely does not cross the placenta. There are no human studies looking at the safety of methacholine in pregnancy. Advantages obtained from testing during the pregnancy should be weighed against the potential risks.
Effect of pregnancy on asthma
The course of asthma is usually unpredictable in pregnancy and numerous studies have suggested that one-third of patients improve, one-third remain the same and the last third worsen [6]. Factors contributing to improvement may be the pregnancy-associated rise in serum cortisol, an anti-inflammatory hormone, or the increase in progesterone which acts as a potent smooth muscle relaxant. However, more is known in terms of factors that predispose to worsening of asthma during pregnancy. There is clear evidence linking upper airway and nasal symptoms and asthma control. The course of asthma seems to parallel that of gestational rhinitis and those patients who have an improvement in their symptoms of rhinitis during pregnancy also have improvements in their asthma symptoms [7]. These findings suggest that the same systemic factors may be affecting both upper and lower airways. In addition, the rate of bacterial sinusitis is 5–6 times higher in pregnant women and may contribute to worsening of asthma symptoms. Gastroesophageal reflux disease (GERD), common in pregnancy, may also play a role in worsening asthma control during pregnancy both through reflux of gastric acid into the airway and through a reflex bronchoconstriction that can occur following acidification of the lower esophagus.
How hormonal factors related to pregnancy affect asthma control is not as well understood. There are many studies of premenstrual asthma that suggest changes in beta-agonist receptor density in the airways that occur during the menstrual cycle. Declines in FEV1 have been shown to occur in the luteal phase in women with premenstrual exacerbations of their symptoms. Emergency room visits were more frequent in the premenstrual period than in the pre-, peri- or postovulatory periods of the menstrual cycle but these findings were not consistent in other studies that showed more visits in the preovulatory phase of the cycle. Unfortunately, however, this information does not translate directly into pregnancy and the presence of premenstrual asthma does not necessarily suggest that asthma will worsen during pregnancy.
Epidemiologic studies done in both the US and Finland suggest that asthma exacerbations are most common between gestation weeks 17–24 [8] and symptoms worsen mostly between 29 and 32 weeks [9]. There is usually an improvement in symptoms after 36 weeks. It is possible that this improvement late in the pregnancy is related to cortisol levels at term reaching four times pre-pregnancy levels.
Of those patients who have worsening of their asthma during pregnancy, close to 60% improve in the postpartum period, whereas worsening was seen in 87% of women whose asthma had improved during pregnancy [10]. When women were followed during successive pregnancies, only 60% followed the same course in the second pregnancy as in the first. Thus, it is difficult to predict with certainty the course asthma will take in an individual pregnancy.
Effect of asthma on pregnancy
Case–control studies have shown that well-controlled pregnant asthmatics do not have a significantly higher rate of adverse outcomes than women without asthma [11,12]. Pregnancy outcomes are not as favorable in women with severe or poorly controlled disease. Of 37,000 women with asthma and 2495 exacerbations, those with exacerbations were more likely to have miscarriage or therapeutic abortions than those without [13]. Suboptimal control appears to be associated with low birthweight, intrauterine growth restriction, and cesarean section. Other adverse pregnancy outcomes thought to be associated with asthma include preterm delivery and maternal hypertension but these risks have not been shown consistently and systemic steroid use may have a confounding effect.
Pre-eclampsia has also been associated with severe asthma in some studies, but it is unclear whether it is the underlying disease or the concomitant use of systemic steroids and co-morbidities that is the culprit [14].
The manner by which poorly controlled asthma affects obstetric outcomes remains unclear. While maternal hypoxia is often offered as an explanation, the majority of pregnant women with even poorly controlled asthma are unlikely to have chronic hypoxia to the degree that would explain these obstetric outcomes. A relationship between obstetric outcomes and chronic inflammatory mediators associated with poorly controlled asthma is therefore speculated although it remains unproven.
Classification of asthma severity
The National Asthma Expert Panel Report (EPR 3) [15] classifies asthma into severity according to two domains – impairment and risk. The term “mild intermittent” has been eliminated and the classification now falls into the following four categories: intermittent, mild persistent, moderate persistent, and severe persistent. Patients within each category can be classified as well controlled, not well controlled or poorly controlled (Tables 1.1 and 1.2). These same categories should be used in evaluating pregnant women with asthma and are useful in directing appropriate management with step therapy.
Table 1.1 Severity assessment and initial treatment
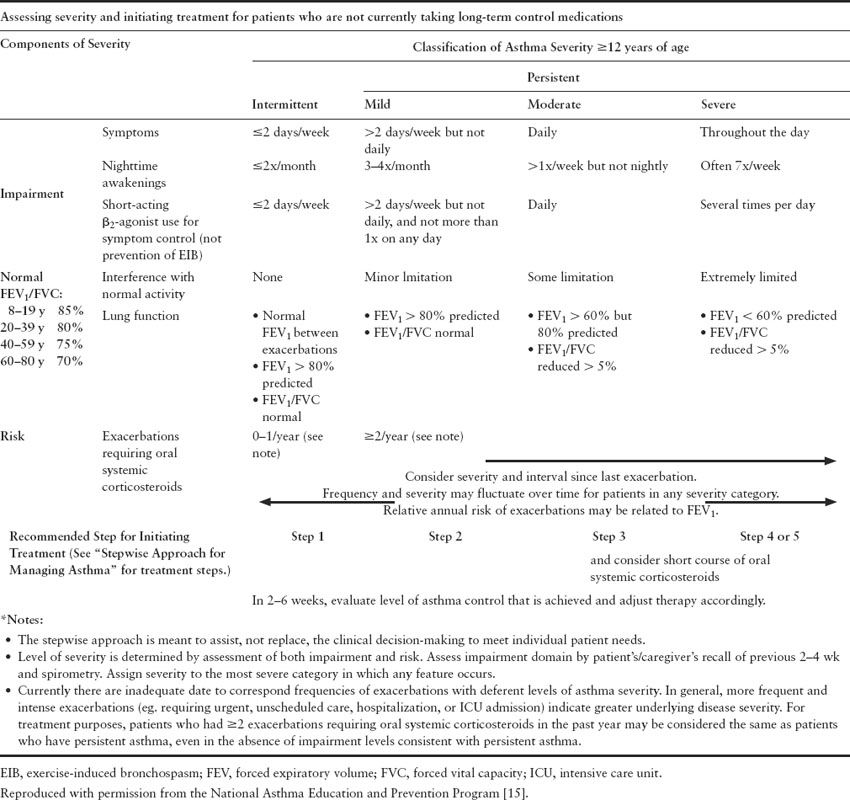
Table 1.2 Control classification
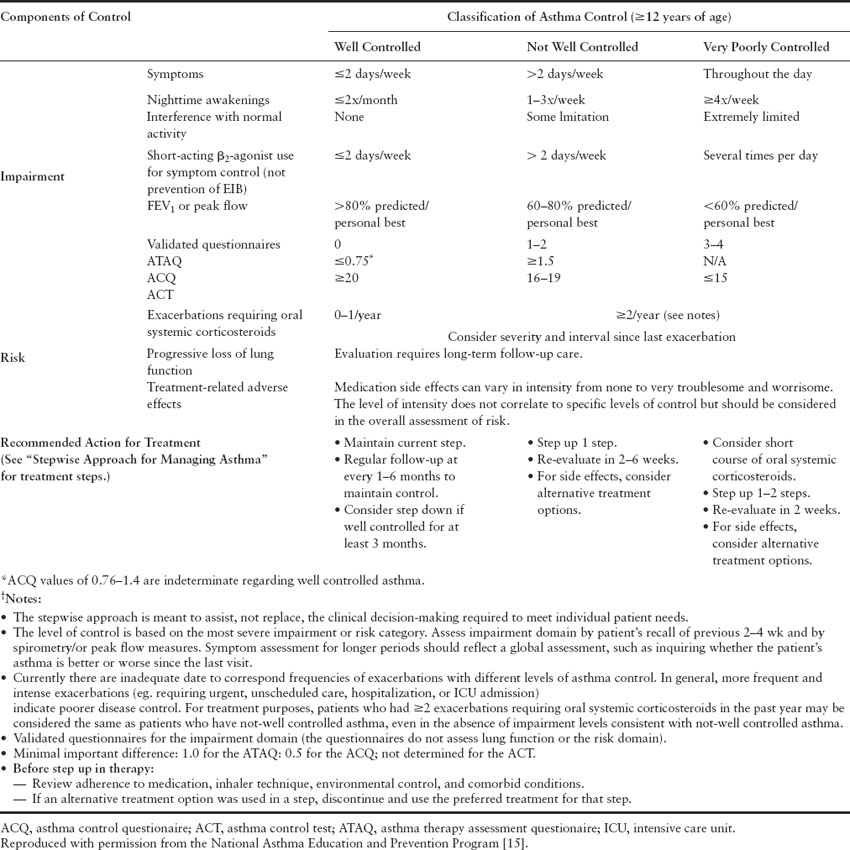
The advantage of the new guidelines compared to the previous guidelines is the fact that risk is now an important feature of the disease severity classification. Need for emergency room visits, frequency of exacerbations and steroid tapers are now taken into account when assessing disease severity. Furthermore, the more recent guidelines reinforce the need to monitor quality of life by using validated measures and emphasize the need for objective measurements with spirometry as part of the initial evaluation and follow-up care.
Management
The first step in management is establishing the diagnosis of asthma. Many patients are misdiagnosed with asthma for many years before they are correctly diagnosed with asthma mimics such as chronic obstructive lung disease, sinus disease or vocal cord dysfunction. Review of medical records and ensuring that the diagnosis is accurate and the history is suggestive are crucial steps.
Another important step is the identification of triggers. Once those have been recognized, every effort should be made to avoid exposure to known triggers. Frequent vacuuming of carpeted areas, avoiding contact with stuffed toys, and using a mattress cover to avoid exposure to dust mites should be encouraged.
According to the US national health interview survey in 2005, 21% of women of childbearing age in the US smoke. Many of those patients are likely to be asthmatic and it is paramount to address smoking habits with every patient. All smokers should be encouraged to quit especially since smoking is the most modifiable risk factor for adverse pregnancy outcomes. Counseling regarding the ill effects of smoking on asthma and fetal health should be done periodically. Strong statements such as: “As your clinician, I need you to know that quitting smoking is the most important thing you can do to protect your baby and your own health” help send a clear message to the pregnant smoker (see section on smoking cessation below).
In addition, pregnant asthmatic patients should be asked about sulfite (additives to prepared foods that preserve freshness) and aspirin sensitivity, rhinitis and sinusitis, GERD, exercise- or cold-induced asthma, and nocturnal asthma. Many pregnant women have GERD so that counseling about lifestyle modifications, including elevating the head of the bed, eating smaller meals, and eating earlier in the evening, can be helpful. Identification and treatment of sinus disease or nasal congestion secondary to allergic rhinitis or gestational rhinitis may also help control symptoms.
Compliance and proper use of medications is another major issue in patients with asthma as poor asthma control results in many cases from inadequate use of the drugs. Pregnancy poses an additional challenge since patients may not be compliant with their medications because of fear of fetal harm. This fear may be propagated by family members, friends, or even other healthcare providers who are less familiar with treating pregnant women. Counseling regarding the safety of the drugs should be done with all asthma patients and it is important to clarify that the benefits of asthma control far outweigh the risks of medications. Reviewing inhaler techniques with all patients should be done not only on their first visit but also periodically on follow-up visits. It should never be assumed that patients who have had asthma for many years know how to properly use their drugs. In our experience, many of those patients use their inhalers incorrectly (Box 1.1).
The National Asthma Education and Prevention Program (NAEPP) has placed substantial emphasis on patient education to help with disease monitoring. In essence, asthma patients need to be educated about their disease, the triggers, ways to avoid them, identifying signs of an attack, monitoring of peak flows and understanding the implications of different stages of obstruction suggested by peak flow meters. In our practice, a written asthma management plan is provided to every patient with asthma after proper education.
Drugs
General principles regarding drug prescription in pregnancy include:
- finding out whether the condition is self-limited and how necessary the medications are;
- evaluating the possible outcomes to the mother and fetus of the untreated condition
- assessing safety data of the drug to be administered and whether other drugs with a better safety profile and similar efficacy could be used instead
- understanding how the patient’s value system and cultural beliefs affect decision making with regard to taking medications during pregnancy.
Box 1.1 Instructions for proper inhaler use
- Inhalers come as metered dose inhalers (MDI), MDI with a spacer, and dry powder inhalers. The latter two have the advantage that hand–breath co-ordination is not critical. The disadvantage of dry powder inhalers, which is not a problem with MDI, is that they do require patients to be able to take a deep, fast breath to get the medication and that an accidental exhalation will blow medication away.
- To use an MDI (e.g. albuterol or salbutamol), shake the inhaler 5–6 times. Remove the mouthpiece cover and place the spacer over the mouthpiece if a spacer is being used. If you are using a spacer, place the lips and teeth over the spacer. If a spacer is not being used, hold the inhaler mouthpiece just outside your open mouth. Breathe in slowly while giving a single squeeze to the top of the canister. Continue to inhale slowly and deeply even after the squeeze has been completed. When you have completely inhaled, hold the breath for 10 seconds. Repeat this procedure in a minute to administer a second “puff.”
- To use a dry powder inhaler (e.g. Pulmicort®), there is no need shake the inhaler. Twist the cover off. “Load” the medication by twisting the base grip to the right as far as it will go and then twist it back to the left. A click should be heard, meaning the medication is loaded. Place the inhaler between your lips in a horizontal position and take a fast, deep breath through your mouth and not your nose, continuing to inhale deeply. Repeat this procedure in a minute to administer a second “puff.”
- To use a dry powder disk inhaler (e.g. Advair®), there is no need to shake the inhaler. Hold the disk level in one hand. With the other hand, put the thumb in the appropriate notch and push it away from you as far as it will go to expose the mouthpiece and the lever for “loading” the medication. Move the lever as far as it will go. A click should be heard, meaning the medication is loaded. Place the mouthpiece between your lips and take a fast, deep breath through your mouth (not your nose) and continue to inhale deeply. Repeat this procedure to administer a second “puff.”
In a patient with asthma, the answer to the first two questions is clear: the attacks should not be assumed to be self-limited and the disease can be life threatening. Therefore the need for therapy is obvious. Below, we will review safety data regarding all the medications used in asthma. Individual counseling should be undertaken in patients with different beliefs and cultural influences to explain the need for therapy and the downside of withholding therapy. The NAEPP published guidelines on pharmacologic management of pregnant patients with asthma in 2004 after reviewing data on fetal safety of the drugs [16].
Short-acting beta-agonists
Short-acting beta-agonists, also called rescue inhalers, are the most potent and rapidly acting bronchodilators currently available for clinical use. Beta-agonists interact with beta-receptors on the surface of a variety of cells implicated in asthma pathogenesis. Among other things, beta-agonists have the potential to relax bronchial smooth muscle and affect vascular tone and edema formation. These drugs have not been shown to affect the maternal circulation even at high doses and are thought to be safe to use in pregnancy.
Long-acting beta-agonists
Formoterol and salmeterol are available in the US. Their safety profile and toxicologic data are similar to those of short-acting beta-agonists. Animal data were suggestive of possible teratogenic effect in one animal species, later labeled as “sensitive rabbits,” when salmeterol was used intravenously at very high doses. However, inhaled use of the drug results in minimal absorption. Further animal studies have not shown such effects even at doses 1600 times higher than the human dose. The use of long-acting beta-agonists is certainly justifiable in pregnancy in patients who are poorly controlled on inhaled corticosteroids alone.
Anticholinergics
Anticholinergic drugs such as ipratropium bromide lead to parasympathetic blockade and further accentuate the bronchodilating effect of beta-agonists. Anticholinergic drugs can be of use in acute exacerbations in the emergency room or hospital (and are often given in combination with beta-agonists in this setting) but have not been shown to have any benefit in long-term therapy of asthma. Although the published data about the safety of these agents in pregnancy are scarce, the systemic absorption is minimal and their use for exacerbations leading to hospital visits is readily justifiable.
Inhaled corticosteroids
Inhaled corticosteroids (ICS) should be the first alternative in patients with poorly controlled symptoms on beta-agonists. Corticosteroids have been shown to inhibit multiple cell types such as mast cells, eosinophils and basophils as well as mediator production and secretion (e.g. histamine and cytokines) involved in asthma pathogenesis. Beclomethasone and budesonide are the most studied inhaled corticosteroids in pregnancy and are thought to be safe for use. However, patients who have been well controlled on a different inhaled steroid prior to conception may be maintained on their drugs since the goal in asthma therapy is optimal control and especially since ICS should be the first-line controller medication.
Leukotriene inhibitors
Leukotrienes are substances that induce numerous biologic activities including augmentation of neutrophil and eosinophil migration, neutrophil and monocyte aggregation as well as increasing capillary permeability and smooth muscle contraction. All these effects contribute to the inflammation, edema, bronchoconstriction, and mucus secretion seen in asthma. Leukotriene inhibitors block the physiologic effects of leukotrienes.
Animal data regarding these drugs suggest that they are likely to be safe for use in pregnancy; however, human data are lacking. Therefore, the risks and benefits need to be balanced in an individual patient. For instance, those patients who required a leukotriene inhibitor in addition to their steroid inhaler and long-acting beta-agonist for optimal symptom control would need to remain on all their drugs during the pregnancy, including leukotriene inhibitors. Patients who are on a leukotriene inhibitor without adequate inhaled steroids may be switched to steroids since more data regarding those are available in pregnancy.
Other drugs
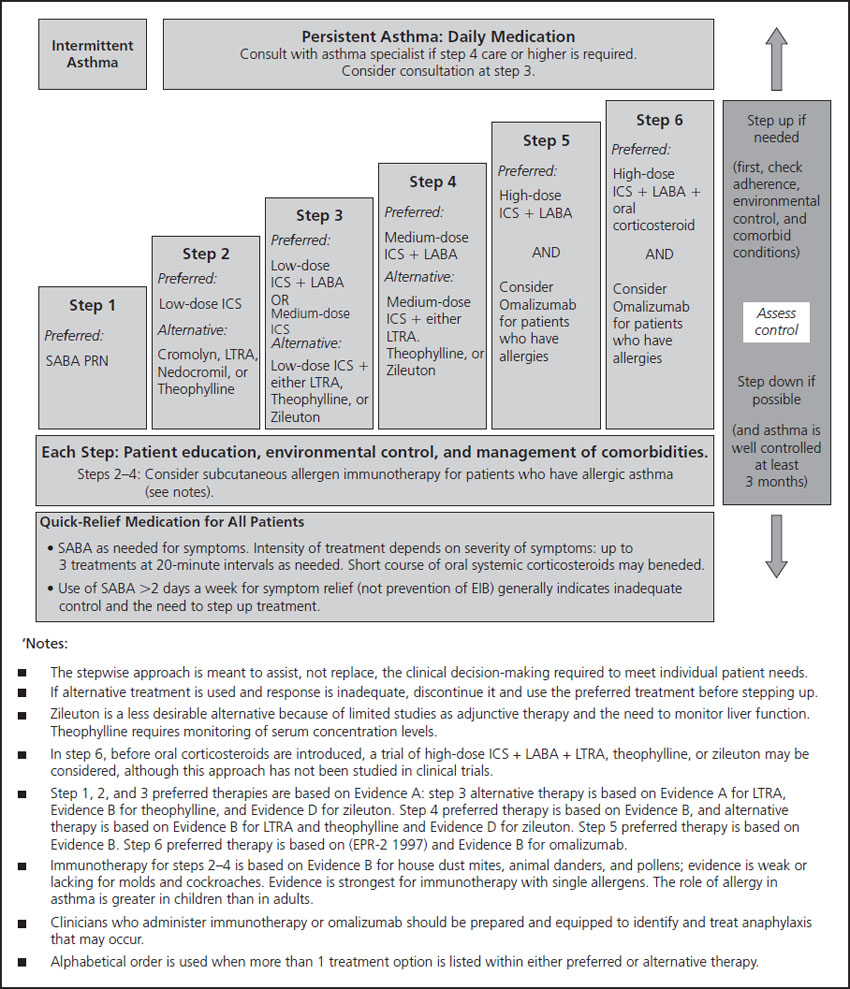
In the most recent report from the National Institutes of Health (NIH) on asthma management in pregnancy, the NAEPP guidelines [16] suggest using low-dose inhaled corticosteroids as a preferred agent in patients with mild persistent asthma, with accepted alternatives listed alphabetically: cromolyn, leukotriene receptor antagonists or theophylline (Figure 1.2). In our experience and in many studies, use of inhaled steroids is certainly superior to the use of any of these agents in the general population. In addition, a study comparing a low-potency steroid (beclomethasone) with theophylline in pregnancy has shown comparable benefit but the steroids were much better tolerated [17]. We believe that inhaled steroids should certainly be used first especially because a superior benefit may be expected from higher potency inhaled steroids compared to beclomethasone, making them a better choice than theophylline.
Figure 1.2 Step therapy for asthma. Reproduced with permission from the National Asthma Education and Prevention Program [15]. EIB, exercise induced bronchospasm; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LTRA, leukotriene receptor antagonist; PRN, as needed; SABA, short-acting beta agonist.
Other studies have suggested that the use of systemic steroids in pregnant asthmatics increases the risk of orofacial clefts (a 2–7-fold increase in risk to 2–14 per 1000 births with use in the first trimester), pre-eclampsia, premature rupture of the membranes and the delivery of both preterm and low birthweight children [14], despite the fact that 87% of prednisone is metabolized by the placenta before it reaches the fetus. Systemic steroids may also contribute to the development of gestational diabetes. However, if indicated, the benefit of systemic steroids to treat inadequately controlled asthma certainly outweighs these risks.
The monoclonal antibody omalizumab blocks the binding of IgE to the IgE receptors and is now being used in moderate to severe asthmatics who are not well controlled on the usual regimen and have significant allergic triggers to their disease. Animal data do not seem to show significant teratogenicity but there have been no safety data in human studies since the initial trials have excluded pregnant patients. Postmarketing data are also limited given that the drug was only recently introduced to the market. For those reasons, the use of omalizumab cannot yet be recommended in pregnancy.
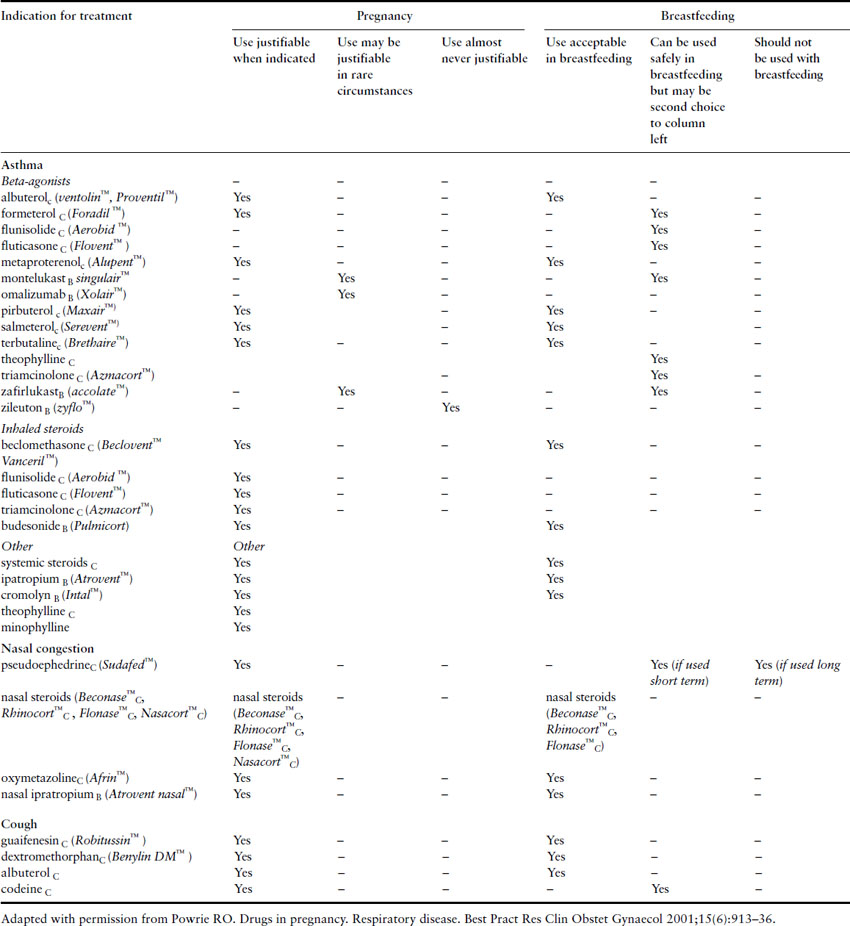
Cimetidine, ranitidine, and metoclopramide can all be used safely in pregnant women with GERD who need pharmacologic treatment. Rhinitis in the pregnant woman can be treated with nasal ipratropium and inhaled nasal steroids. See Table 1.3 for an overview of the drugs used to treat asthma in pregnancy. It is also recommended that all pregnant women receive immunization for influenza regardless of gestation and this is particularly important in asthmatics (see influenza section below).
Table 1.3 Drugs for treating common pulmonary conditions in pregnancy
Management of acute exacerbations
Patients presenting with an acute exacerbation should be assessed promptly. Those with a clear history of asthma and an exam suggesting an acute exacerbation should receive bronchodilators without delay. Peak flow measurements help determine the severity of the attack, guide therapy, and monitor for a response to interventions (Box 1.2). On physical exam, patients should be assessed for the use of accessory muscles since this suggests a severe exacerbation. Arterial blood gases should be obtained if patients are not improving with initial treatment or if a severe exacerbation is suspected. It is important to recognize that normal PaCO2 in pregnancy is 30–35 mmHg. Therefore, a tachypneic patient with a PaCO2 above that range should prompt the suspicion of impending respiratory failure.
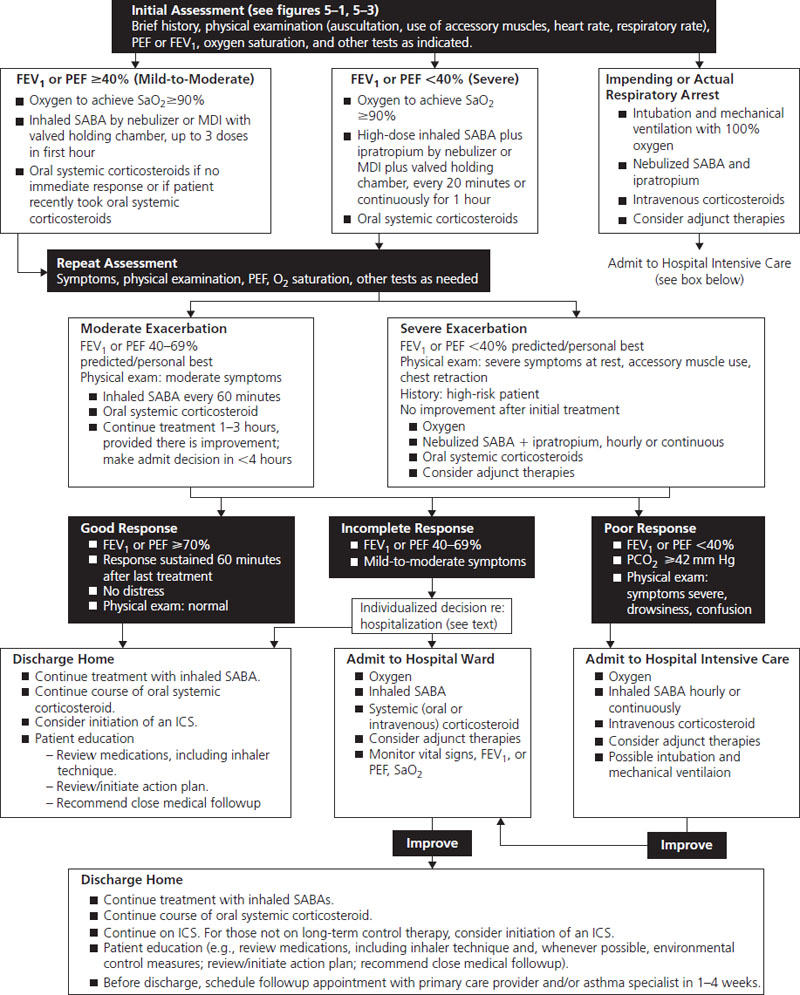
The NAEPP guidelines published in 2004 have clear advice on the management of acute exacerbations of asthma in pregnancy and are shown in Figure 1.3. It should be noted that this is essentially unchanged from treatment in nonpregnant patients.
Figure 1.3 Management of asthma exacerbations: emergency department and hospital-based care. Reproduced with permission from The National Asthma Education and Prevention Program [15]. FEV1, forced expiratory volume in 1 seconds; ICS, inhaled corticosteroid; MDI, metered dose inhaler; PCO2, partial pressure carbon dioxide; PEF, peak expiratory flow; SABA, short-acting beta-agonist; SaO2, oxygen saturation.
Fetal surveillance during pregnancy
The primary affect on the fetus from asthma, or any other pulmonary disease, is chronic hypoxia. The impact of hypoxia can manifest in several ways, including growth restriction or, more significantly, fetal death. Shortly after a woman with asthma becomes pregnant, she should have an early ultrasound to confirm her pregnancy dating. Women should be instructed to monitor fetal activity during the course of the pregnancy. A third-trimester ultrasound can be considered in a woman with well-controlled asthma who has appropriate growth in the fundal height. The NAEPP Working Group recommends serial ultrasounds starting at 32 weeks gestation in women with suboptimally controlled asthma and women with moderate-to-severe asthma [16]. If the growth is not appropriate or the woman has an acute exacerbation, fetal testing should be started. Testing may include umbilical artery Doppler flow velocity studies, nonstress testing (NST) or biophysical profiles (BPP). The frequency of such testing would depend on the severity of the patient’s asthma or the degree of growth restriction.
Labor and delivery
Asthma exacerbations are rare in labor and delivery. This is thought to be related to the increase in serum cortisol that occurs during that period. Despite that, it is advisable to administer stress doses of steroids to patients who have been on prolonged systemic steroids during the pregnancy (see Chapter 47). Asthma medications should not be discontinued through labor and delivery.
Prostaglandin E2 is safe for cervical ripening, as is oxytocin. The agent 15-methyl prostaglandin F2-alpha should be avoided because it may cause severe bronchospasm. Although methylergonovine may cause dyspnea, asthma is not an absolute contraindication, and therefore it can be used when appropriate in the management of postpartum hemorrhage. Fentanyl is preferred to morphine and meperidine, which can release histamine. Epidural anesthesia is usually advised because it decreases oxygen consumption and minute ventilation. Epidural anesthesia also decreases the possibility of requiring general anesthesia if an emergency cesarean becomes indicated during labor. However, if cesarean delivery is required, a high level of sensory block may produce some degree of patient anxiety in the intraoperative period.
Several published articles in recent years have suggested that the increase in cesarean delivery rates over past years may be linked to the increasing incdence in asthma in the general population [18,19]. The “hygiene hypothesis” is put forward as a possible explanation as the establishment of GI flora in neonates born by cesarean section is delayed and this could have implications for the development of the neonatal immune system which ultimately leads to atopy and asthma. However, the existence of a relationship between asthma and mode of delivery requires further exploration in prospective studies that are controlled for confounding variables at the time of this writing.
Postpartum period
During the postpartum period, women should initially continue the same asthma medications they required during pregnancy. Close peak flow monitoring is indicated, particularly in those with poorly controlled or moderate-to-severe asthma. Most drugs used for asthma treatment can be safely used in breastfeeding women (see Table 1.3). In fact, breastfeeding should be encouraged given the many well-recognized benefits for both mother and baby. Whether breastfeeding decreases the likelihood of the development of asthma in offspring is as yet controversial but it does appear to decrease atopy. The need for medication compliance should be reinforced as some mothers will find it more difficult to tend to their own needs when they have a newborn. Women who have quit smoking during their pregnancy are at increased risk for returning to their old habit so a discussion focused on maintaining abstinence may be useful. (See smoking cessation section below.)
Box 1.2 Use of the peak flow meter
- Peak flow meters are inexpensive portable, hand-held devices that measure the patient’s ability to push air out of her lungs and are used as a convenient standardized way for a patient and her provider to monitor the course of her asthma on a daily basis. Peak flow meters also help identify worsening asthma before the patient may be aware of an exacerbation and thereby allow early intervention.
- Patients should measure their peak flow rate around the same time each day, often first thing in the morning and early in the evening. Normal peak flow rates can be obtained in standardized charts and vary with height, gender and race. A 30-year-old white woman with a height of 5’ 5” will typically have a peak flow rate of 400 L/min. Most experts, however, will have their patients measure themselves against their own “personal best,” i.e. the best peak flow rate that they have obtained under stable conditions. Patients can be taught to consider peak flow rates within 80% of their personal best as “normal” (the “green zone”), flow rates that are within 50–80% of normal as cause for concern or caution (the “yellow zone”) and flow rates less than 50% of normal as being potentially dangerous (the “red zone”). Patients should be given explicit instructions as to what they should do with results in each zone.
Smoking cessation in pregnancy
The health risks of cigarette smoking outside pregnancy are well established and include atherosclerotic cardiovascular disease, cancers of the lung, cervix, pancreas, kidneys, lower urinary tract, and upper digestive tract, as well as respiratory illness including chronic obstructive pulmonary disease and worsening of asthma. It is also associated with diseases such as osteoporosis and peptic ulcer disease. As the leading preventable cause of death worldwide, it is responsible for approximately 1 in 10 deaths in adults and costs billions of dollars in annual health-related economic losses. For the smoking pregnant woman and her fetus, the risks are more immediate and include low birthweight, spontaneous pregnancy loss, stillbirth, premature rupture of membranes, placental abruption, placenta previa, and preterm delivery.
Epidemiology
According to studies from the 1990s, between 1 in 3 and 1 in 5 women living in developed countries reported smoking during pregnancy [20]. However, the prevalence may be even higher as smoking is notoriously under-reported by gravid women, probably because of the lack of social acceptability of cigarette use in pregnancy. Smoking is particularly common in those who are socially disadvantaged and have low income but poor social support, depression, work stress, and exposure to intimate partner violence are also associated factors. Women with concern about weight gain during pregnancy may use continued smoking as a method of weight control.
Pathophysiology
Smoking is the most important modifiable risk factor for adverse pregnancy outcome. It is estimated that in a population with a high smoking prevalence, smoking cessation could prevent up to 10% of perinatal deaths, 35% of low birthweight babies, and 15% of preterm deliveries [21]. Mechanisms by which these adverse outcomes may occur include impaired oxygen delivery to the fetoplacental unit, exposure to carboxyhemoglobin, direct fetal genetic damage or other toxicities from the multiple substances present in cigarettes and cigarette smoke. In the postpartum period, cigarette smoking is associated with further risks for babies including an increased risk of neonatal death and sudden infant death syndrome, respiratory infections, asthma, otitis media, colic, childhood obesity, and possibly type 2 diabetes mellitus (Box 1.3).
Management
Antepartum
The benefit of smoking cessation both within and outside pregnancy is clear but successful abstinence is difficult. However, factors which may help motivate pregnant women include the desire to have a healthy pregnancy and newborn and the frequent contact with healthcare providers who can provide tobacco abstinence encouragement and support. Complete smoking cessation is only accomplished by approximately 20–40% of smoking pregnant women. Of those who do stop, most have already quit by their first prenatal visit. Risk factors for continued smoking include lower education status, those smoking less than 10 cigarettes per day, having a partner who smokes, and those who have other psychosocial issues. A study looking at a group of women who continued to smoke during pregnancy cited the following reasons for continued smoking: skepticism about smoking-related harms, addiction to nicotine, smoking among partners/family members, doubt about the safety of nicotine patch, and that the provider stopped asking about smoking status [22].
Box 1.3 Adverse pregnancy outcomes associated with smoking
- Infertility
- Low birthweight
- Spontaneous pregnancy loss
- Stillbirth
- Premature rupture of membranes
- Placental abruption
- Placenta previa
- Preterm delivery
- Possibly congenital malformations
Adverse effects on neonates and infants of mothers who smoke
- Increased risk of neonatal death and sudden infant death syndrome
- Increased respiratory infections (including otitis media)
- Increased asthma
- Increased colic
- Possible increased childhood obesity
- Possible increased type 2 diabetes mellitus
The 5As (Ask, Advise, Assess, Assist, Arrange) remain the cornerstone in approaching smoking pregnant women just as for nonpregnant patients (see Box 1.3, Tables 1.4 and 1.5). Inquiries about smoking status should be made on each visit and appropriate reinforcement given. If a patient is considering smoking cessation, a discussion centered on her continued need for stopping and recommendations for possible cessation strategies should be provided. Once a patient has actually stopped, continued positive reinforcement of useful strategies will be helpful for ongoing success. Additional resources for specific strategies for smoking cessation in pregnancy will be found at the following websites: www.modimes.org, www.helppregnantsmokersquit.com, and www.acog.org.
Table 1.4 The 5 As for pregnant women
Adapted with permission from the National Partnership to Help Pregnant Smokers Quit: www.helppregnantsmokersquit.org.
| The 5 As | Action | Length of time spent |
| ASK about smoking status | Ask the pregnant woman to describe herself as one of the following: (a) I have NEVER smoked or have smoked LESS THAN 100 cigarettes in my lifetime (b) I stopped smoking BEFORE I found out I was pregnant, and I am not smoking now (c) I stopped smoking AFTER I found out I was pregnant, and I am not smoking now (d) I smoke some now, but I cut down on the number of cigarettes SINCE I found out I was pregnant (e) I smoke regularly now, about the same as BEFORE I found out I was pregnant | 1 minute |
| ADVISE quitting | Give the patient strong, clear advice to quit smoking describing the impact of smoking and the benefits of quitting on the mother and fetus (see Table 1.5) | 1 minute |
| ASSESS willingness to quit | Discuss whether the patient is willing to quit smoking in the next 30 days | 1 minute |
| ASSIST in helping patient to quit | Discuss problem-solving methods and skills for cessation. Provide pregnancy-specific self-help materials. Encourage social support in the smoker’s environment | 3 minutes + |
| ARRANGE follow-up | Periodically assess smoking status and encourage cessation if continued smoking | 1 minute |
Table 1.5 Benefits of smoking cessation in general over time
Adapted with permission from the National Partnership to Help Pregnant Smokers Quit: www.helppregnantsmokersquit.org.
| Time period | Result |
| Within 20 minutes | Blood pressure drops to near that of before the last cigarette. Temperature of hands and feet increases to normal |
| Within 12 hours | Carbon monoxide level drops to normal |
| Within 24 hours | The risk of myocardial infarction decreases |
| Within 2–3 weeks | Circulation improves and lung function increases |
| Within 1–9 months | Coughing, sinus congestion, fatigue, and shortness of breath decrease |
| Within 1 year | The excess risk of heart disease is half that of a smoker’s |
| Within 5 years | The risk of stroke reduces to that of a nonsmoker’s |
| Within 10 years | The risk of many cancers decreases, including lung, mouth, and throat cancer |
| Within 15 years: | The risk of heart disease reduces to that of a nonsmoker |
Drugs
In nonpregnant women, pharmacotherapy is strongly encouraged but because of concerns regarding drug use in pregnancy, medication is often not considered as a tool for use in smoking cessation in pregnancy. However, the considerable risks of ongoing tobacco use in a gravida unable to stop smoking without pharmacotherapy must be balanced against the risks of medication use. In particular, nicotine has known adverse fetal effects for which it has earned an US Food and Drug Administration (FDA) pregnancy safety category D (“studies have demonstrated a risk to the fetus”). However, with continued smoking, a fetus is exposed not only to nicotine but also to many other substances with adverse effects. Interestingly, several recent studies have suggested no worsened and even improved fetal outcome associated with nicotine replacement therapy. One study looking at the rate of stillbirth in pregnant women using nicotine gum, patch or inhaler did not show an increase in stillbirth in nicotine replacement users [23]. The use of 2 mg nicotine gum in a study by Oncken et al. did not show increased quit rates but did show increased birthweight and a lower risk of preterm delivery as compared with placebo [24]. Ideally a pregnant woman would stop smoking without any pharmacologic aids but in the practical world, the likelihood is lower. Therefore, many would consider the benefit of nicotine replacement therapy and the higher likelihood of smoking cessation to be greater than the risk of continued smoking, especially in women who are at high risk for continued smoking.
Though bupropion is more effective for smoking cessation than nicotine replacement therapy outside pregnancy, there are limited data on its use in pregnancy for either smoking cessation or depression. Therefore, nicotine replacement is preferable for use in pregnancy. Both nicotine replacement and bupropion are acceptable for breastfeeding but nicotine replacement is preferable. Likewise, the safety of varenicline in pregnancy and lactation is even less clear and it should be avoided in pregnant and breastfeeding women until more data are available.
Post partum
Among those women who do stop smoking during pregnancy, 90% will relapse in the first postpartum year and most often within the first 6 weeks after delivery. Therefore, cessation programs should target this time period to minimize recidivism. Discussions addressing postpartum relapse should begin in the third trimester (Box 1.4). Risk factors for relapse include women with depressed mood, women who have family members or friends who are continued smokers, those with less social support, and those with less confidence in their ability to remain smoke free. Women who smoke in the postpartum period are also less likely to breastfeed.
Box 1.4 Specific benefits of smoking cessation to tell pregnant patients
- After you stop smoking more nutrition will go to your baby to help him/her grow.
- After you stop smoking, your chances of having a healthy baby increase, and the baby is more likely to have a healthy childhood.
- After you stop smoking you will have more energy and may feel less stressed.
- After you stop smoking you’ll breathe easier and you will be better able to keep up with your active, healthy baby.
- After you stop smoking, you’ll reduce your risk for cancer, cardiovascular, and other diseases so you can be around a long time to be a good mother.
Adapted with permission from You and your baby, American Lung Association: www.lungusa.org.
Rhinitis and sinusitis
Up to 30% of women develop symptoms of rhinitis or sinusitis during pregnancy and the risk dramatically increases in smokers [25]. The effects of increased blood volume and vascular congestion on the nasal mucosa are thought to be responsible for gestational rhinitis. In addition, women with underlying allergic rhinitis or nasal polyps may develop worsening of their baseline symptoms with pregnancy.
Rhinitis may be categorized as allergic or nonallergic, both presenting with similar symptoms of rhinorrhea and nasal obstruction but with varied underlying causes. Allergic rhinitis may not be life threatening but its negative impact on quality of life of pregnant women is significant. Allergic rhinitis often co-exists with asthma and up to 40% of rhinitis patients become asthmatics [26]. Furthermore, clinically diagnosed allergic rhinitis is associated with worse asthma control. Of note, rhinitis in pregnancy may be associated with snoring.
Reassurance may be the only intervention necessary for rhinitis but women with particularly bothersome symptoms can try saline nasal spray or intranasal preparations of beclomethasone and/or cromolyn [27]. First-generation antihistamines such as chlorpheniramine or diphenhydramine are reasonably used to treat allergy-related symptoms. Warning against overuse of topical decongestants which can cause rhinitis medicamentosa is important as many women may try these to avoid systemic medications. Table 1.6 outlines the differential diagnosis of rhinitis in pregnancy with recommended treatment options.
Table 1.6 Differential diagnosis of rhinitis in pregnancy and some treatment options
| Diagnosis | Clinical features | Treatment |
| Rhinitis medicamentosa | A condition of rebound nasal congestion brought on by extended use of topical decongestants (e.g. oxymetazoline, phenylephrine, and xylometazoline nasal sprays) that work by constricting blood vessels in the lining of the nose | Discontinue topical vasoconstrictors |
| Pregnancy rhinitis | Runny nose and nasal congestion presenting at any time in pregnancy caused by mucosal and vascular changes in nasopharynx | Buffered saline nose spray or nasal lavage, external nasal dilator (e.g. Breath-Rite strips) |
| Infectious rhinosinusitis | Usually a bacterial infection complicating the common cold with symptoms worsening after 5 days or not improving after 10 days and nasal congestion, fever and sinus pain predominating | Antibiotics (usually amoxicillin or azithromycin in penicillin-allergic patients) if no co-morbid conditions and no recent prior antibiotic use), nasal lavage, limited use of oxymetazoline nasal spray or pseudoephedrine after first trimester |
| Vasomotor rhinitis | Itch, sneeze, cough, nasal congestion, rhinorrhea in response to specific allergens (e.g. hay fever) | No specific therapy. Nasal ipratropium or pseudoephedrine after first trimester for limited period |
| Allergic rhinitis | Nasal congestion and rhinorrhea in response to nonallergenic environmental stimuli (e.g. cold, heat, alcohol, emotion, spicy food) | Intranasal cromolyn +/− nasal oxymetazoline or pseudoephedrine, topical beclomethasone or fluticasone, oral antihistamines (chlorpheniramine maleate, diphenhydramine) |
Sinusitis is increased sixfold in pregnancy as compared with nonpregnant patients. Complicating its diagnosis is the fact that 50% of pregnant women with documented purulent sinusitis do not have classic sinusitis symptoms (sinus tenderness, purulent discharge, fever). Therefore, despite the common rhinitis symptoms of pregnancy, clinicians should have a high index of suspicion for sinusitis in gravid women. The organisms causing sinusitis in pregnancy are the same as in the general population so antibiotics should be geared towards covering Haemophilus influenzae, Mycoplasma. catarrhalis, and Streptococcus pneumoniae. Assuming antibiotic resistance patterns do not dictate otherwise, reasonable antibiotic choices for use in pregnancy include amoxicillin/clauvulanate, cefuroxime axetil, and azithromycin. However, ciprofloxacin, doxycycline and tetracycline should be avoided.
Acute bronchitis
Acute bronchitis usually refers to a self-limited respiratory illness characterized by the predominance of a productive cough in a patient with no history of chronic obstructive pulmonary disease and no evidence of pneumonia. It affects approximately 5% of adults in the US annually [28].
Most cases of acute bronchitis seem to have a viral etiology; however, atypical bacteria including Bordetella pertussis, Chlamydia pneumoniae and Mycoplasma pneumoniae are important causes [29]. The etiologic pathogen is isolated from the sputum in only a minority of patients.
During the first few days of infection, the illness is indistinguishable from other acute upper respiratory infections. However, with acute bronchitis, coughing persists for more than 5 days, and during this period the results of pulmonary function testing may become abnormal [30]. Reduction in FEV1 or bronchial hyper-reactivity may be noted, with improvement in the following 5–6 weeks. Typically, cough persists for 3 weeks following acute bronchitis, but may last 4 weeks or more.
Differential diagnosis includes asthma, bronchiolitis, bronchiectasis or acute exacerbation of chronic bronchitis. Chronic bronchitis by definition is the presence of cough and sputum production on most days of the month for at least 3 months of the year during 2 consecutive years.
No studies have looked specifically at the course of acute bronchitis in pregnancy. One retrospective cohort study found an association between placental abruption and acute respiratory illnesses, including acute bronchitis among white women [31].
Most patients with acute cough syndromes require no more than reassurance and symptomatic treatment. A chest X-ray would only be indicated if pneumonia was suspected on clinical exam. Diagnostic testing for a particular pathogen can only be justified when the organism is treatable and a community outbreak is suspected.
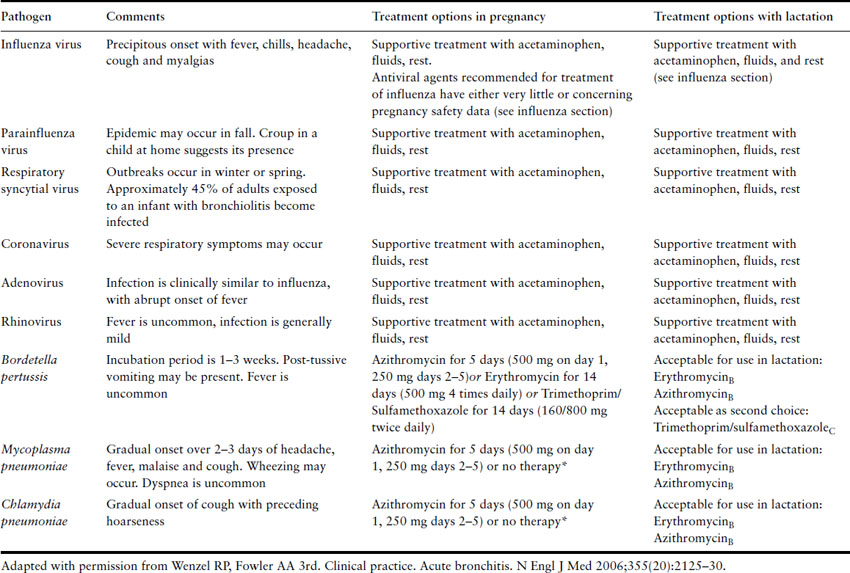
Antimicrobial agents are not recommended in most cases of acute bronchitis. Multiple studies indicate that patients with acute bronchitis do not benefit from these drugs. Antimicrobial therapy may be considered in patients when a treatable pathogen is identified or in epidemic settings to limit transmission. Table 1.7 includes suggested treatment regimens for pregnant patients.
Table 1.7 Recognized causes of acute bronchitis and treatment options
Pneumonia
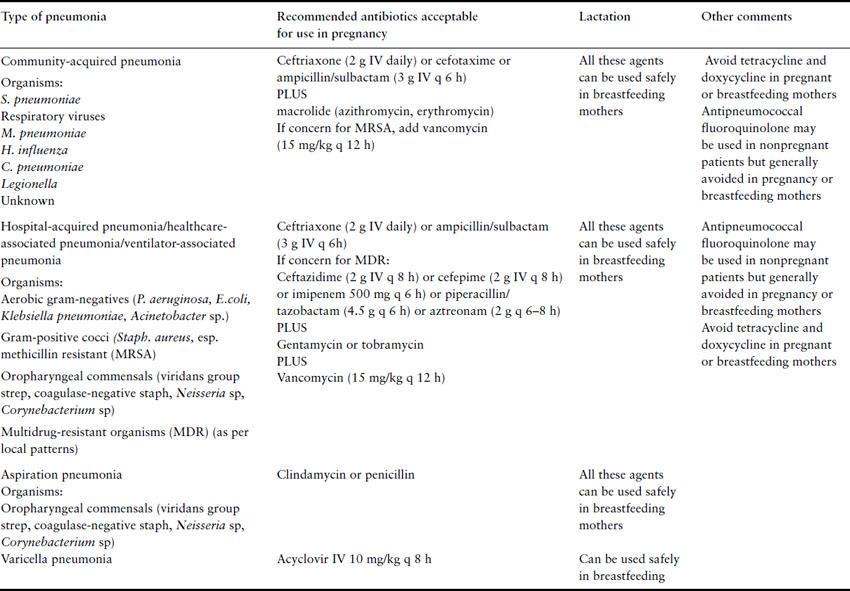
Pneumonia and influenza combined are the seventh leading cause of mortality in the United States and the most common cause of death from an infectious disease [32]. The incidence of pneumonia requiring hospitalization in pregnancy is between 2.6 and 15.1 per 10,000 deliveries, a rate comparable to that seen in nonpregnant women of a similar age [33]. Although historically pneumonia has been cited as the third most frequent cause of indirect obstetric death in North America, several recent studies have reported no or rare maternal deaths, with mortality rates similar to young hospitalized nonpregnant patients [34]. Despite this, pregnancy is associated with reduction in cell-mediated immunity and this may explain the increased risk of severe pneumonia and disseminated disease from atypical pathogens such as herpes virus, influenza, varicella, and coccidioidomycosis in pregnant women. Other anatomic and physiologic changes of pregnancy that may add to the vulnerability of the lung to injury during infection include an increase in thoracic circumference, elevation of the diaphragm (resulting in interference with clearance of secretions), decreased functional residual capacity, and increased oxygen consumption. This section will discuss the various types of pneumonia (Table 1.8).
Table 1.8 Types of pneumonia and their treatment
Bacterial and atypical pneumonia
Although rigorous investigation into specific causes of pneumonia in pregnancy is lacking, the etiology is likely similar to the nonpregnant population, with Streptococcus pneumoniae being the most commonly isolated organism. Other causes of pneumonia include Staphylococcus aureus, Haemophilus influenzae, Legionella spp, Mycoplasma pneumoniae, Chlamydia and viruses. Among patients requiring admission to intensive care units, Pseudomonas aeruginosa and Enterobacteriaceae also play an important role. However, even with extensive diagnostic testing, the etiologic agent cannot be identified in at least 50% of cases.
Clinical presentation
Signs and symptoms of pneumonia in pregnancy are similar to those in nonpregnant individuals. Symptoms usually include cough, sputum production, chills, rigors, dyspnea and pleuritic chest pain, although nonrespiratory symptoms such as vomiting, abdominal pain and fever may also occur. Physical exam may reveal fever, tachypnea, hypoxia, abnormal breath sounds, including a pleural friction rub, egophony (consolidation causing the patient’s spoken “a” to sound like an “e” on auscultation) or tactile fremitus (consolidation causing the spoken words “99” to cause a palpable vibration on the chest wall). Hemodynamic instability may be present in cases of severe illness. Mothers who develop pneumonia are more likely to have co-existing medical problems including asthma, drug abuse, anemia and HIV infection. The use of corticosteroids for enhancement of fetal lung maturity and tocolytic agents has also been associated with antepartum pneumonia [35].
Diagnosis
Information obtained from the history or physical examination cannot rule in or rule out the diagnosis of pneumonia with adequate accuracy. Therefore, to confirm the diagnosis and to assess severity of illness and presence of complications such as pleural effusion or multilobar disease, a chest radiograph should be performed in all patients suspected to have pneumonia. Laboratory data should include a complete blood count, serum chemistries for hepatic, renal and glucose evaluation, assessment of oxygenation and two sets of blood cultures; however, blood cultures may be positive only 7–15% of the time. The American Thoracic Society (ATS) does not recommend routine performance of sputum culture and gram stain. However, if a drug-resistant pathogen or an organism not covered by usual empiric therapy is suspected, sputum culture should be obtained. HIV status should be reviewed for all pregnant women with pneumonia and testing should be offered if it has not previously been done. Testing for Pneumocystis jiroveci infection should occur in all HIV-positive women who present with pneumonia.
The differential diagnosis for a pregnant woman presenting with symptoms of pneumonia is varied. Pulmonary embolism can present identically to an acute pneumonia with dyspnea, cough, chest pain, low-grade fever and chest X-ray infiltrates and remains the leading direct cause of maternal mortality in the US and the UK. Aspiration chemical pneumonitis, amniotic fluid embolism and pulmonary edema related to sepsis, tocolysis or pre-eclampsia can also present in a similar fashion. Other infectious illnesses, including cholecystitis, appendicitis and pyelonephritis, should also be considered.
Management
Preconception counseling
Pneumonia does not generally prompt preconception counseling because it is an acute infectious illness. There are a few issues worth consideration, however. Women who are HIV infected with low CD4 cell counts should continue prophylaxis for Pneumocystis jiroveci (see Chapter 18). Immunizations to prevent pneumonia and its complications are also indicated. The Centers for Disease Control and the American College of Obstetricians and Gynecologists advise that women should routinely receive influenza vaccination (see section on influenza below). Women with diabetes mellitus, asthma, chronic cardiac or pulmonary disease, chronic hypertension or immune compromise disease should receive the pneumococcal vaccine. It is also recommended post splenectomy and in women with functional hyposplenism, such as with sickle cell disease, and for women living in prisons or long-term care facilities. All nonpregnant women of childbearing age who are not immune to varicella should be vaccinated but it is a live vaccine and should not be given during pregnancy.
Potential maternal and fetal complications
Pregnancy increases the risk of maternal complications from pneumonia, including the need for mechanical ventilation. Respiratory failure due to pneumonia is the third leading indication for intubation in pregnancy [36]. Other maternal complications include pulmonary edema, bacteremia, empyema and pneumothorax. Pregnancies complicated by acute respiratory illnesses, including viral and bacterial pneumonia, have been shown to be associated with placental abruption [31]. Increased rates of preterm labor and delivery before 34 weeks of gestation have also been described [37], resulting in significantly lower average birthweight at delivery. The neonatal mortality rate due to antepartum pneumonia ranges from 1.9% to 12%, with most mortality attributable to complications of preterm birth [38]. Although most cases of pneumonia in pregnancy are caused by organisms which do not affect the fetus except through their effects on maternal status, some organisms, such as varicella, may present specific risks to the fetus. The fetus may also be at risk from maternal conditions which predispose to pneumonia, such as anemia or HIV infection.
Treatment
Although several guidelines to assess severity and need for hospitalization have been developed for pneumonia in the nonpregnant population, discriminatory features that identify those pregnant women who can be successfully managed as outpatients have not been determined. Pregnant women with pneumonia should generally be admitted for initial therapy, fetal evaluation and to ensure adequate oxygenation (oxygen saturation ≥95% or pO2 ≥70 mmHg).
Several recommendations for the empiric treatment of community-acquired pneumonia exist. These support the use of a macrolide (erythromycin in any form except estolate ester, or azithromycin) in conjunction with a beta-lactam (cefotaxime, ceftriaxone or ampicillin-sulbactam) for most inpatients with pneumonia. Although levofloxacin and doxycycline are often recommended in the treatment of pneumonia in the nonpregnant population, these drugs should be avoided in pregnancy. Clarithromycin has shown adverse effects in animal trials at doses equivalent to 2–17 times the maximum recommended human dose. It is therefore best avoided in pregnancy, with use limited to those cases where no alternative therapy is appropriate. Monotherapy with high-dose amoxicillin (3–4 g a day) is supported in some European recommendations for nonpregnant patients, but ATS guidelines suggest addition of azithromycin for adequate coverage of H. influenzae (see Table 1.8).
With appropriate antibiotic therapy, some improvement in the patient’s clinical course should be seen within 72 hours. Patients initially treated with intravenous antibiotics can be switched to oral agents (erythromycin/azithromycin with cefprozil or cefpodoxime) once the patient is afebrile for 24–48 hours. Continuation of therapy for a total of 10–14 days is recommended for all agents except azithromycin which can be given for only a 5-day course because of its extended half-life. Clinicians should ensure that a follow-up chest X-ray is done to confirm that there is no other underlying pathology complicating the pneumonia.
Aspiration pneumonia
Due to significant progress in modern obstetric and anesthetic management, acute aspiration pneumonitis has become a very uncommon cause of pneumonia in pregnancy. Although aspiration usually occurs in association with a difficult intubation or during the postanesthetic period when the gag reflex may be depressed, it may also develop de novo in pregnant women. Gastric juice in the lungs leads to intense pulmonary inflammation over 8–24 hours. The patient becomes tachypneic, hypoxic and febrile and the chest X-ray can show a complete “white out.” Despite this rapidly deteriorating course, the picture resolves without antibiotics within 48–72 hours unless bacterial superinfection intervenes.
Bacterial aspiration pneumonia usually has a more insidious onset. Clinical manifestations typically begin 48–72 hours after aspiration, with persistent fever, sputum and leukocytosis. In this syndrome, chest X-ray findings are typically localized to the basilar segments (if the patient aspirated while upright) or to the posterior segment of the upper lobe or the superior segment of the lower lobe (if the patient aspirated while supine). The bacterial infection is generally polymicrobial with mouth anaerobes predominating and antibiotic treatment with penicillin or clindamycin is recommended.
Viral pneumonia
Varicella and influenza are the most common pathogens associated with viral pneumonia in pregnancy; however, cases with pneumonia resulting from rubella, hantavirus and SARS have also been reported in pregnancy. Viral pneumonia is often complicated by acute respiratory failure, secondary bacterial infections and acute respiratory distress syndrome (ARDS). This chapter will cover influenza, severe acute respiratory syndrome (SARS), and varicella pneumonia.
Influenza in pregnancy (see also Chapter 17)
Outbreaks or epidemics of influenza generally occur in the fall and winter but can occur year round in the tropics. The viruses causing influenza are of two types, A and B. The A virus is further classified by the hemagglutinin (H) and neuramidase (N) surface antigens and antibodies against these antigens decrease the likelihood of infection. However, antibodies to one subtype do not necessarily confer immunity to another or a variant of a subtype, such as occurs with antigenic drift. This is an important consideration in determining the influenza strains against which the annual vaccines are targeted. A major change in the antigens resulting in essentially a novel influenza virus, termed antigenic shift, has the potential to cause pandemics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree