Materials and Methods
This is a secondary analysis of the Randomized Clinical Trial of Magnesium Sulfate for the Prevention of Cerebral Palsy conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The details of this study have been previously reported. Briefly, this was a multicenter, randomized, placebo-controlled study conducted at 20 institutions across the United States from 1997 to 2004 with the aim of determining whether antenatal magnesium sulfate administration to women at high risk for preterm delivery decreased the rate of cerebral palsy or death.
In this study, women were exposed to magnesium sulfate if they were at imminent risk for delivery between 24 and 31 weeks because of the rupture of membranes, advanced preterm labor, or fetal indications. In this protocol, magnesium was discontinued after 12 hours if delivery had not occurred and was no longer considered imminent. Magnesium was restarted when delivery was again deemed imminent. As a result, not all women who were exposed to magnesium sulfate were receiving the medication at the time of preterm delivery.
For the current study, women with singleton gestations were included. Those assigned to the placebo group or with major fetal malformations, stillbirth, or missing outcome data at 2 years were excluded. Study groups were defined by time interval from the last magnesium sulfate exposure to delivery: exposure to magnesium sulfate <12 hours prior to delivery, including at the time of delivery, and exposure ≥12 hours prior to delivery. If magnesium sulfate was discontinued and restarted, the time from the last exposure to delivery was used.
The primary outcome was any type of cerebral palsy, assessed and diagnosed at or beyond 2 years of age (corrected for prematurity) by an annually certified pediatrician or pediatric neurologist if 2 or more of the following 3 features were present: a delay of ≥30% in gross motor developmental milestones; abnormality in muscle tone, ≥4 or absent deep tendon reflexes, or movement abnormality; persistence of primitive reflexes or absence of protective reflexes.
When cerebral palsy was diagnosed, the Gross Motor Function Classification System was used to assess severity (mild, Gross Motor Function Classification System level 1; moderate, Gross Motor Function Classification System level 2 or 3; or severe, Gross Motor Function Classification System level 4 or 5). Secondary outcomes included the following: (1) moderate and severe cerebral palsy and (2) moderate and severe cerebral palsy or death.
Baseline characteristics among the cohorts were compared using a Student t test, Mann-Whitney U test, and χ 2 tests as appropriate. Incidences of the primary and secondary outcomes were compared between the 2 groups. Unadjusted relative risks with 95% confidence intervals were estimated. Multivariable logistic regression models were developed to estimate the risk of each outcome based on timing of magnesium sulfate, adjusting for confounding factors that were identified based on historical importance and in the bivariate analyses with a value of P < .05. These included maternal race, education level, prenatal care, nulliparity, preeclampsia, antibiotic exposure, total grams of magnesium sulfate received, mode of delivery, gestational age at delivery, and neonatal sepsis.
Using goodness-of-fit testing, the model that best fit the data was chosen as the final model. This model included magnesium sulfate as a categorical rather than a continuous variable. All tests were 2 tailed with P < .05 considered significant. Because our sample size was fixed from the parent trial, we evaluated the detectable effect size in this cohort. Based on the total sample size, rate of exposure, and rate of outcome, assuming an alpha of 0.05 and beta of 0.20, our study had sufficient statistical power to detect a 2-fold increased risk for the primary outcome of any cerebral palsy. SAS software, version 9.4 (SAS Institute, Cary, NC) was used for the analysis.
This analysis was considered exempt by the Institutional Review Board at Columbia University Medical Center because these are publicly available, deidentified data.
Results
Of the 2444 infants included in the original trial, 1256 were exposed to placebo and thus excluded. The remaining exclusion criteria, including multiple gestation, fetal anomalies, antenatal stillbirths, and missing 2 year follow-up information, were then applied in a sequential fashion, leaving a final sample of 906 infants ( Figure ).
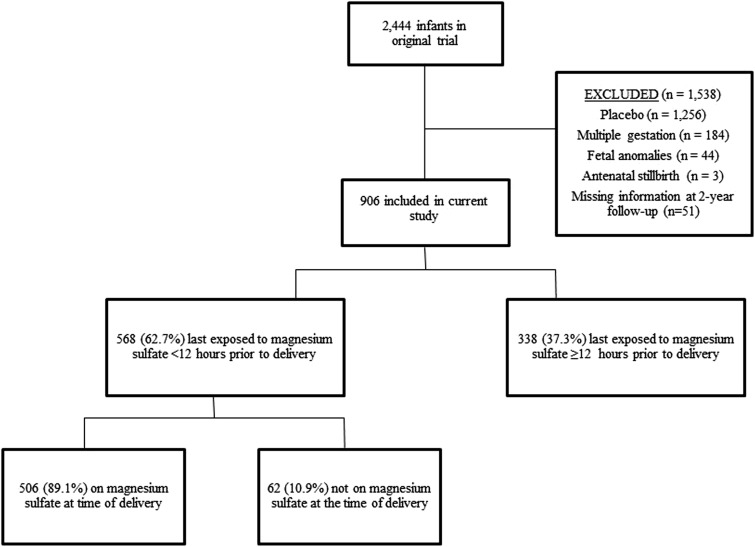
A total of 568 infants (62.7%) were last exposed to magnesium sulfate <12 hours prior to delivery, of which 506 (89.1%) had magnesium sulfate infusing at the time of delivery. Three hundred thirty-eight (37.3%) were last exposed to magnesium sulfate ≥12 hours prior to delivery. Overall, cerebral palsy occurred in 28 offspring (3.1%), 2.3% of those last exposed <12 hours, and 4.4% last exposed ≥12 hours prior to delivery.
There were significant differences between the groups in several characteristics, including education level, prenatal care, nulliparity, preeclampsia, antibiotic exposure, gestational age at delivery, preterm birth, and birthweight ( Table 1 ). There were also significant differences in the amount of magnesium sulfate received, hours on magnesium sulfate, whether magnesium sulfate was infusing at delivery, and the time interval between last magnesium sulfate exposure to delivery.
| Characteristics | Last magnesium exposure <12 h prior to delivery (n = 568) | Last magnesium exposure ≥12 h prior to delivery (n = 338) | P value |
|---|---|---|---|
| Maternal age, y b | 26.21 ± 5.82 | 26.78 ± 5.80 | .16 |
| Advanced maternal age (≥35 y) | 58 (10.21) | 38 (11.24) | .63 |
| Race | |||
| African-American | 251 (44.19) | 152 (44.97) | .79 |
| White | 203 (35.74) | 125 (36.98) | |
| Hispanic | 99 (17.43) | 56 (16.57) | |
| Asian | 7 (1.23) | 3 (0.89) | |
| Native American/other | 8 (1.41) | 2 (0.59) | |
| Marital status | |||
| Married | 281 (49.56) | 171 (50.59) | .94 |
| Single | 230 (40.56) | 133 (39.35) | |
| Unknown | 56 (9.88) | 34 (10.06) | |
| Body mass index, kg/m 2 | |||
| <18.5 | 98 (17.25) | 46 (13.61) | .24 |
| 18.5–24.9 | 233 (41.02) | 151 (44.67) | |
| 25–29.9 | 116 (20.42) | 59 (17.46) | |
| >30 | 121 (21.30) | 82 (24.26) | |
| Education, y b | 11.64 ± 2.49 | 12.01 ± 2.51 | .03 |
| Prenatal care | 539 (94.89) | 306 (90.53) | .01 |
| Smoking | 154 (27.11) | 91 (26.92) | .95 |
| Alcohol use | 44 (7.75) | 33 (9.76) | .29 |
| Recreational drug use | 62 (10.92) | 32 (9.47) | .49 |
| Nulliparous | 224 (39.44) | 98 (28.99) | .002 |
| Previous preterm delivery | 155 (27.29) | 95 (28.11) | .79 |
| Diabetes | 27 (4.75) | 19 (5.62) | .57 |
| Preeclampsia | 3 (0.53) | 7 (2.07) | .03 |
| Membranes ruptured | 475 (98.75) | 308 (99.04) | .71 |
| Antenatal corticosteroid exposure | 23 (4.20) | 15 (4.50) | .83 |
| Antibiotic exposure | 502 (88.38) | 314 (92.90) | .03 |
| Chorioamnionitis | 67 (11.80) | 36 (10.65) | .60 |
| Sepsis | 100 (17.61) | 53 (15.68) | .45 |
| Total magnesium infused, g c | 39.60, 29.30–51.20 | 29.30, 29.20–30.00 | .001 |
| Total magnesium infused, h c | 14.90, 12.10–20.50 | 12.00, 12.00–12.30 | .001 |
| Magnesium sulfate on at delivery | 506 (89.08) | 0 (0) | .001 |
| Hours since last magnesium sulfate infusion to delivery c | 0, 0–0 | 169.30, 66.00–516.40 | .001 |
| Outcomes | |||
| Gestational age at delivery in weeks b | 29.36 ± 2.48 | 30.61 ± 3.72 | .001 |
| Preterm birth (delivery <37 wks) | 568 (100.00) | 321 (94.97) | .001 |
| Vaginal delivery | 376 (66.20) | 210 (62.13) | .22 |
| Male gender | 324 (57.04) | 183 (54.14) | .40 |
| Birth weight, g b | 1342.9 ± 443.0 | 1582.6 ± 704.4 | .001 |
| Any cerebral palsy | 13 (2.29) | 15 (4.44) | .07 |
| Moderate and severe cerebral palsy or death | 52 (9.15) | 26 (7.69) | .45 |
a Data are presented as percentage unless otherwise indicated
Adjusting for maternal race, education level, prenatal care, nulliparity, preeclampsia, antibiotic exposure, total grams of magnesium sulfate received, mode of delivery, gestational age at delivery, and sepsis, the last exposure to magnesium sulfate <12 hours prior to delivery was associated with a significant reduction in cerebral palsy compared with women last exposed ≥12 hours (adjusted odds ratio, 0.41, 95% confidence interval, 0.18–0.91) ( Table 2 ).



