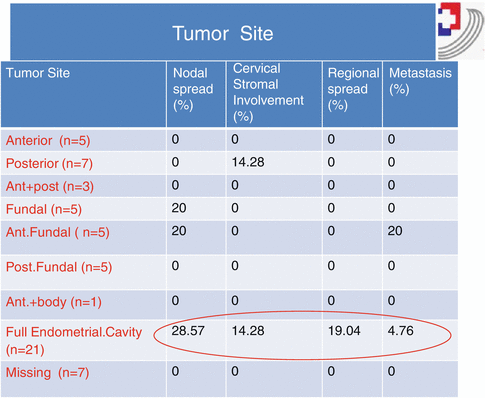
Stage
5 years survival (%)
1A
88
1B
75
11
69
111A
58
111B
50
111C
47
1v A
17
1v B
15
Types of the Tumor
There are two types of tumors.
Type I tumors usually occur in pre- and perimenopausal women, often with a history of unopposed estrogen exposure and/or endometrial hyperplasia. They are often minimally invasive into the underlying uterine wall and are of low grade endometroid type and carry a good prognosis.
Type II tumors occurs in older, postmenopausal, thin women, and are not associated with increased exposure to estrogen; they are more aggressive and less differentiated and carry a poor prognosis. These include clear cell tumor, papillary serous tumors, and carcinosarcomas. These tumors have mainly p53 mutation and ERBb-2 (her 2 neu) expression.
Myometrial Invasion
Depth of myometrial invasion, tumor extension to the cervix, and lymph nodal status are part of FIGO staging, each of the above factor involvement progressively upstages the disease and they are independent prognostic factors themselves. Increasing depth of myometrial infiltration is associated with increasing tendency to extrauterine spread. Superficial or no myometrial infiltration is seen with well differentiated tumors. Deep myometrial invasion is seen frequently in poorly differentiated and undifferentiated tumors and thus is an alarming sign for lymph nodal involvement and distant metastasis and is often independent of degree of differentiation [4, 5]. Patients with >50 % involvement of myometrium is associated with poor prognosis. Patients whose myometrium has not been involved do not have much lymph-vascular space invasion even [6].
Deep myometrial involvement often coexists with cervical involvement by endometrial adenocarcinomas and has an adverse effect on prognosis [7]. Patients with lower uterine segment involvement are more likely to have pelvic and paraarotic nodal disease, and increasing local recurrence [8]. Spread to lymph nodes is associated with poor prognosis and require adjuvant treatment.
Tumor Size
In majority of tumors, T stage includes tumor size, the larger the tumor the more advanced the stage and lesser the survival. In endometrial carcinoma, increasing T stage indicates increasing depth of uterine wall infiltration. However many authors could correlate increasing tumor size with poor outcome in uterine carcinomas [8]. The conventional threshold is a measure of 2 cm [9]. Some have attempted to quantify three-dimensional tumor volume and correlate this risk to metastatic spread and survival [10].
Tumor Site
Tumor location inside the uterus can predict distant nodal disease and indicate chance of recurrence. Tumor involving fundal region has increased risk of paraaortic lymph node involvement. Tumor occupying the whole endometrial cavity significantly upstages the cancer [11] (Fig. 12.1, and Table 12.1).
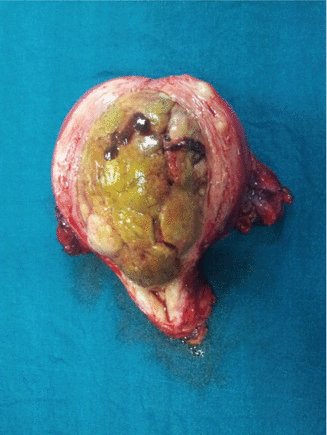

Fig. 12.1
Tumor occupying whole of the endometrial cavity
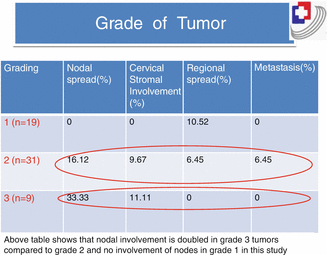
Grade of the Tumor
Since long, grade of the tumor has been regarded as an important prognostic factor in endometrial cancer [12]. Adenocarcinomas having 5 % or less nonsquamous or nonmural solid growth are designated as grade 1, those with 6–50 % solid growth as grade 2, and those with more than 50 % solid growth as grade 3. The 5-year survival rate in stage 1 carcinoma endometrium depends on the grade; the higher the grade, the poorer the prognosis (Tables 12.2 and 12.3)
Table 12.2
Five-year survival in stage 1 endometrial cancer
Grade | Surgical (%) | Clinical (%) |
|---|---|---|
1 | 93 | 60 |
2 | 90 | 50 |
3 | 79 | 29 |
Peritoneal Cytology
Positive peritoneal cytology portends a poor prognostic factor in earlier studies [1, 13]. The impact on survival of positive peritoneal cytology in the absence of other extrauterine disease is unclear and the treatment aimed at this is not well founded [14]. The following mechanisms may be deduced from the literature for the presence of malignant cells in the peritoneal cavity [15, 16]. (1) Result of transtubal transport; (2) direct extension of tumor through the myometrium; (3) lymphatic metastasis to the peritoneal cavity; and (4) reflection of multifocal peritoneal occult spread. Transtubal transport seems to be the most logical and popular. In more recent studies the authors are of opinion that the presence of positive peritoneal cytology is not an independent prognostic factor, and that it does not seem to reflect the potential of peritoneal spread in patients with endometrial carcinoma confined to the uterus [17, 18]. Positive peritoneal cytology is removed from FIGO Staging now; however it should be documented separately.
Patients having adnexal metastasis and peritoneal implants have poor prognosis as they indicate extrauterine spread and have more chances of pelvic and paraaortic lymph nodal involvement.
Age
Endometrial cancer occurs rarely in women under the age of 40. Most cases are found in women aged 50 and over, with more than half of the cases diagnosed in the age group of 50–69. The risk of endometrial cancer increases as the woman gets older. Age is not a significant variable of outcome after adjusting for other poor prognostic factors [19]. One study [20] divided patients to two groups, age in Group A was 59 years (range 50–69) and Group B was 75 years (range 70–92). Patients in Group B were more likely to have hypertension and coronary artery disease. There were no differences in progression-free or disease-specific survival; however, Group B had a worse overall survival proved to be due to associated comorbidities.
Many studies addressed the value of race as a prognostic factor in carcinoma endometrium [21–23]. Analysis of 41,120 cases of endometrial cancer indicated that race was a prognostic factors in addition to FIGO stage, histology, histologic grade, lymph node status, and age at diagnosis [21]. When incorporating the number of poor prognostic factors in a survival model with race and surgical stage, race ceased to be of significant prognostic value [22]. Although the incidence of endometrial cancer is less in Black women, cancer specific survival rates were lower in them when compared to that in white women. This racial difference in survival is multifactorial and include later diagnosis, treatment disparities, comorbid conditions, and genetic differences which result in the occurrence of more aggressive tumors in Black Americans [23].
DNA Ploidy
In a recent study [24], predictive and prognostic factors were analyzed in a consecutive series of 4543 endometrial carcinomas and it was concluded that DNA ploidy was an independent and significant prognostic and predictive factor. Eight predictive and prognostic factors were analyzed in this study with regard to recurrence and survival. The factors analyzed were: age, FIGO stage, histology, FIGO grade, nuclear grade, DNA ploidy, myometrial infiltration, and p53 expression. The 5 years actuarial locoregional recurrence rate was 3.6 %, the factors which independently affected the recurrence rate were FIGO grade, DNA ploidy, and depth of myometrial infiltration. The 5 years actuarial overall survival rate in these patients was 73 % and cancer specific survival was 83 %. All the factors studied except p53 expression analyzed with immunohistochemistry were found to be significantly affecting overall and cancer specific survival rates. Tumor stage was the single most important factor with a risk ratio of 4.2 followed by FIGO grade 2.5 and 1.6 for DNA ploidy. Myometrial invasion had the lowest risk ratio of 1.3 in this study with regard to survival.
LVSI
Lymphovascular space invasion is an important predictor for prognosis of disease as these are the patients who are at high risk for recurrences. The risk of pelvic and paraaortic lymph node involvement increases significantly. Gadducci et al. [25] in 2009 reported that their univariate and multivariate analysis on 259 endometroid endometrial cancer patients showed lymphvascular space involvement (LVSI) and deep myometrial invasion as the independent predictive variables for the risk of distant hematogeneous failure. The analysis included 12 patients in stage 1B-2 who developed distant failure compared to 20 randomly chosen control group who were disease free after a median period of 52 months.
In multivariate analysis of 324 high intermediate and high risk endometrial cancer patients (stage 1–3), who came for adjuvant radiotherapy in Maccallum Cancer Centre, for relapse, positive LVSI had a hazard ratio of 4.9, which increased to 8.8 in the presence of positive nodes [26]. For overall survival, only LVSI was significant, with a hazard ratio of 3.02. In particular, in the presence of LVSI and nodes, histological type, grade, and myometrial invasion were not significant factors. Five hundred twenty-five endometrial cancer patients who underwent primary surgery were assessed for the impact of LVSI on recurrence and survival [27].
LVSI in this study was associated with a high risk of recurrence and poor overall survival in early stage endometrial cancer; therefore, it is prudent to include evaluation of lymph vascular space involvement in the clinical decision to decide whether or not a patient with early stage endometrial cancer should receive adjuvant therapy.
Risk group definition [24] is very important in predicting prognosis: apart from pathological factors dna ploidy is also included in this risk categorization.
The definition of high-risk carcinomas was as follows: (1) FIGO stage I, (2) nonendometrioid histological type, (3) presence of two of the following risk factors: FIGO grade 3 (poorly differentiated), deep (≥50 %) myometrial infiltration, DNA aneuploidy (FCM), (4) nuclear grade 3, (5) pathologically negative lymph nodes, and (6) negative abdominal cytology. Points 5–6 were optional in this study, and data are not available for all cases.
The definition of medium-risk carcinomas was as follows: (1) FIGO stage I, (2) endometrioid histological type, (3) presence of one of the following risk factors: FIGO grade 3 (poorly differentiated), deep (≥50 %) myometrial infiltration, DNA aneuploidy (Flow cytometry (FCM)), (4) nuclear grade 1–2, (5) pathologically negative lymph nodes, and (6) negative abdominal cytology. Points 5–6 were optional in this study, and data are not available for all cases. Lymph vascular space invasion (LVSI) was not regularly included in the pathology reports at the participating centers and was not included in the definition of the medium-risk group.
The definition of low-risk carcinomas was as follows: (1) FIGO stage I, (2) endometrioid histological type, (3) presence of none of the following risk factors: FIGO grade 3 (poorly differentiated), deep (≥50 %) myometrial infiltration, DNA aneuploidy (FCM), or (4) nuclear grade 3. All pathology reports were reviewed by one experienced pathologist at the regional referral center.
It is interesting to note that 54 % of all endometrial tumors will come under the low risk category and 22 % will come under high risk category. The low risk and high risk groups significantly differ in their survival outcomes, with the high risk group getting only 50 % cancer specific survival. The risk grouping helps oncologist to discriminate between patients who require surgery alone (low risk), who require surgery plus brachytherapy (intermediate risk), and those who require external beam radiation and chemotherapy in addition to surgery [28–30].
Tumor Markers as Prognostic Factors in Endometrial Carcinoma
CA 125 as a prognostic factor was studied by Espino-strebel and Luna [31] in 90 patients. They concluded that Ca 125 was significantly correlated with deep myometrial invasion, adnexal metastasis, pelvic and paraaortic nodal involvement, and recommended routine preoperative Ca 125 estimation. A receiver operating characteristic curve (ROC) was constructed to determine Ca 125 cutoff value. A cutoff value of 55 U/ml can predict extrauterine spread with sensitivity of 53.85 %, specificity of 84.38 %, and accuracy of 75.56 %.
Denschlag et al. [32] analyzed 101 patients of stage 3 endometrial cancer to find the prognostic factors of treatment outcome. They observed that an elevated Ca 125 level, adnexal involvement, the final tumor grade, and the lymph node dissection were independent predictors of cause-specific survival.
In multivariate analysis of the results of 100 normal subjects, 47 patients with benign gynecological diseases and 97 patients with endometrial cancer [33] found CA15.3 to be highly significant and had a larger hazard ratio. Univariate analyses showed that the increase of all the three, CA125, CA15.3, and CA19.9, were significantly associated with shorter survival.
Biological and Molecular Prognostic Factors
Among the oncogene expressions, the widely studied one is Her-2neu oncogene expression. Hetzel et al. [34] found Her-2neu oncogene’s overexpression to be associated with a poor overall survival. The fraction of cells in S-phase has also been found to be an important prognostic indicator of clinical outcome [35].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree