Series
Miscarriages
Total
Proportion miscarrying (%)
Tongsong et al. [56]
14
255
5.6
Tannirandorn et al. [57]
3
87
3.4
Falco et al. [58]
23
149
15.4
Bennett et al. [38]
48
516
9.3
Subchorionic hematoma
Total
88
1,007
8.7
Weiss et al. [7] enrolled patients into a database on presenting with a viable embryo at 10–14 weeks. If the patient reached 10–14 weeks, the chance of miscarrying prior to 24 weeks was 1–2 %. Another source of ascertainment of the miscarriage rate after threatened miscarriage is from the control group of randomised trials of progestogens in threatened miscarriage. These can be seen in Figs. 4.2 and 4.3. In these nine studies the miscarriage rate was higher. 129 pregnancies of 519 resulted in miscarriage (24.9 %). However, these figures are not corrected for the presence of a fetal heartbeat prior to enrollment in the various trials.
In recurrent miscarriage, vaginal bleeding is a common complication occurring in 50 of 162 women in Reginald’s series [8] and 50 of 102 patients in the author’s series [9]. The reason for this bleeding remains unclear. 75 % of habitual abortions are blighted ova [9]. However, when the pregnancy succeeds and there is a live embryo within the uterus, bleeding still occurs in 40–50 % of patients. The likelihood of a pregnancy loss after the detection of fetal heartbeats as 69/359 (14.2 %) in Li et al.’s series [10] and 22.7 % of 185 study patients with multiple spontaneous abortions in Laufer et al.’s [11] series.
3 Progesterone Levels
Serum progesterone levels have been used to make prognoses about the continued development of pregnancy and even to diagnose pregnancy loss. The lowest progesterone level to be associated with a viable pregnancy was 5.1 ng/ml in Stovall et al.’s [12] series. A single progesterone level ≥25 ng/ml was associated with a 97 % likelihood of viable pregnancy [12]. Al-Sebai et al. [5] summarised 358 threatened abortions <18 weeks, a single progesterone level ≤ 45 nmol/l (14 ng/ml) was reported to differentiate between aborting and ongoing pregnancies (sensitivity 87.6 %, specificity 87.5 %). Serum progesterone levels of less than ≤12 ng/ml. were associated with an increased risk of miscarriage in Arck et al.’s [13] series.
The mechanisms by which luteal phase deficiency occur are unclear but could be associated with decreased FSH levels in the follicular phase (it has been suggested that luteal phase deficiency originates as a preovulatory event). Alternatively there may be an abnormal pattern of LH secretion. Luteal phase deficiency may be secondary to abnormal follicle formation, associated with poor oocyte quality, or a decreased response to progesterone by the endometrium [14]. However, there are pitfalls to using serum progesterone levels as a predictive marker of miscarriage, or for determining the need for progesterone supplementation. Progesterone secretion is pulsatile. Blood may be drawn at a pulse peak or nadir. These may vary tenfold [15]. Hormone levels may be normal, but histology abnormal due to deficiency of progesterone receptors. As with other presumptive causes of abortion, low hormone levels may be a result of abortion rather than its cause. In the blighted ovum or after embryonic death, there is no villous circulation. Trophoblastic failure after villous circulatory failure results in low hCG levels. If hCG does not stimulate the corpus luteum, progesterone levels will fall explaining the mechanism of expulsion, but not necessarily that of embryonic death, or the cause of abortion. Therefore in threatened miscarriage, diagnosis and treatment should be empirical rather than based on progesterone levels.
4 Confounding Factors
The results of progestogen treatment may be confounded as threatened miscarriage, may be due to separation of the placenta in a normal embryo, or a defence mechanism to prevent the continued development of an abnormal embryo, including abnormalities which are incompatible with life. The most important confounding factors are embryonic structural malformations, or chromosomal aberrations. In missed abortions 200 of 233 embryos have been reported to be structurally abnormal on embryoscopy [16]. These defects included: anencephaly, encephalocele, spina bifida, syndactyly, pseudo-syndactyly, polydactyly, cleft hand and cleft lip. Without embryoscopy these embryos would not have been diagnosed, and the patient might have been treated empirically with progesterone. If included in a trial, the results would be skewed in favour of a negative effect. However, embryoscopy is advanced technique, which is not usually available. Additionally, 70 % of miscarriages are blighted ova. Therefore, it is impossible to tell if a rudimentary embryo may have been structurally abnormal. At present, ultrasound is not sufficiently sensitive to diagnose these very early anomalies.
Up to 60 % of sporadic miscarriages [17–19] may be caused by chromosomal aberrations in the embryo. The most common aberrations which are incompatible with life include 16 trisomy and polyploidy. Additionally, with the introduction of comparative genomic hybridization (CGH) a further 15 % of so called normal embryos on conventional karyotyping, have been diagnosed with genetic aberrations [20, 21].
Progesterone cannot correct chromosomal aberrations. Unfortunately, the abortus is not usually karyotyped, even less are subjected to CGH microarray analysis. Hence, it is unclear whether miscarriage after supplemental progesterone is due to failure of treatment or confounding of the results by fetal chromosomal aberrations.
Before a trial of progesterone can be said to be conclusive, other predictive factors should be taken into account.
5 Effect of Progestogen Supplementation
Progestogens have been used since the 1950s to prevent threatened miscarriage terminating as miscarriage. It has been difficult to prove whether progestogens are effective, due to the generally good natural history, and the effect of confounding factors. Recently, two systematic reviews have been performed. Wahibi et al. [22] carried out an analysis of two trials of oral dydrogesterone compared to placebo [23, 24] and two trials of vaginal progesterone [25, 26] (See Fig. 4.1). The overall figures showed a statistically significant benefit, OR = 0.53 (CI 0.35–0.79) in favour of progestogen supplementation. However, the analysis consisted of only four studies. The authors concluded, “The analysis was limited by the small number and the poor methodological quality of eligible studies, and the small number of the participants, which limit the power of the meta-analysis and hence of the conclusion”. It is interesting to note that in the women who were treated with vaginal progesterone the treatment was not statistically effective in reducing miscarriage when compared to placebo (RR = 0.47 95 % CI, 0.17–1.30) whereas oral progestogen (dydrogesterone) was effective (RR = 0.54 CI 0.35–0.84). In the light of Wahibi et al.’s [22] metaanalysis, two new guidelines were released regarding threatened miscarriage. In the U.K. the National Institute for Health and Care Excellence (NICE) [27] produced a guideline in 2012 which stated, that “data from a meta-analysis of several small studies suggest that progestogens are better than placebo”. The Royal Australian and New Zealand College of Obstetricians and/gynaecologists guideline [28] stated that, “Miscarriage was significantly less likely to occur on progestins than placebo or no treatment (risk ratio 0.53; 95 % CI 0.35–0.79)”. Both guidelines stated, that further research was necessary to confirm the results on a larger number of patients.
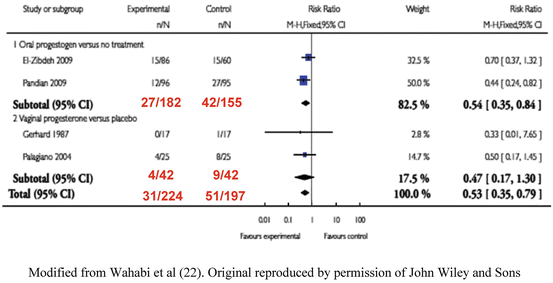

Fig. 4.1
Metaanalysis of RCT’s on progestogen supplementation. Modified from Wahabi et al. [22]. Original reproduced by permission of John Wiley and Sons
The author tried to enlarge the number of patients available for analysis by relaxing the strict methodological rules required by the Cochrane database for inclusion in metaanalyses. A literature search was performed for in September 2010 for all papers available at that time in EMBASE and Ovid MEDLINE® that fulfilled the following criteria: original contributions with product name ‘Progestogen, Progestin, Progesterone, Micronised progesterone Duphaston’ or ‘dydrogesterone’, reports limited to clinical human data excluding reviews, case reports and editorials in any language. All articles considered were investigator initiated trials and published in the scientific literature.
No additional relevant trials were found for micronized or intramuscular progesterone, to those assessed in Wahibi et al.’s [22] metaanalysis. However, three additional trials of oral dydrogesterone were considered eligible for inclusion in a systematic review [29–31]. Carp’s [32] metaanalysis included five randomized studies. 660 patients were eligible for analyses of pregnancy maintenance. The results (Fig. 4.2) showed that the effect of dydrogesterone on the risk of miscarriage in women with threatened miscarriage appeared to be substantial. There was a statistically significant reduction in the odds ratio for miscarriage after dydrogesterone compared to standard care of 0.47 (CI 0.31–0.7). The 24 % miscarriage rate in control women (78/325) was reduced to 13 % (44/335) after dydrogesterone administration (11 % absolute reduction in the miscarriage rate). There has since been a further trial of vaginal micronised progesterone. Yassaee et al [33] have reported on the results of 60 patients treated with threatened miscarriage who were treated with vaginal micronised progesterone. Although there was a trend towards an improved prognosis, the results were not significantly better than in the control group.
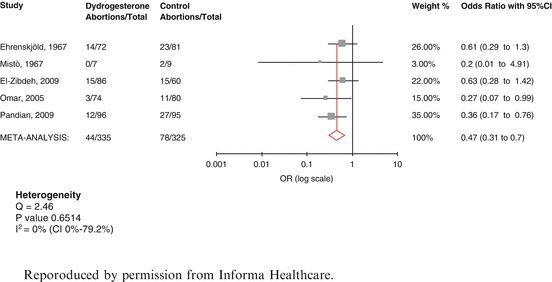

Fig. 4.2
Metaanalysis of dydrogesterone in threatened miscarriage [32]. Reproduced by permission from Informa Healthcare
5.1 Evidence of Effect
There are two metaanalyses in the literature which show progestogens to lower the risk of threatened miscarriage developing to miscarriage. In both metaanalyses the effect of dydrogesterone was statistically significant (Grade I). There a dearth of evidence about 17 hydroxyprogesterone acetate or caproate by intramuscular injection, as only one small trial of been reported to restore levels of soluble TNF receptors to normal in women with recurrent pregnancy loss [34]. However, Shearman and Garrett [35] found 17 hydroxyprogesterone caproate to have no beneficial effect in threatened miscarriage. Additionally, when myometrial tissues were suspended in organ chambers and exposed to varying concentrations of progesterone or 17 hydroxyprogesterone caproate [36] progesterone significantly inhibited spontaneous contractility, but 17 hydroxyprogesterone caproate actually stimulated contractility. It is therefore not recommended for threatened miscarriage. Unfortunately, only two trials on vaginal progesterone were analysed in Wahibi et al.’s [22] metaanalysis. There were only 84 patients in both trials. Although there was a reduction in the number of miscarriages progressing to miscarriage, both trials were not sufficiently powered to reach statistical significance. Palagiano et al. [26], and Yassaee et al, [33] used micronised progesterone. Neither trial reached statistical significance.
Needless to state that no studies control for karyotypic aberrations or predictive factors.
5.2 Subgroups of Threatened Miscarriage
5.2.1 Subchorionic Hematoma
Subchorionic hematoma is a common occurrence in threatened miscarriage, being found in approximately 18 % of all cases of bleeding during the first trimester [37]. There is one observational study on the natural history of subchorionic hematoma in threatened abortion after detection of the fetal heart [38]. The incidence of miscarriage was 8.9 %, similar to other cases of threatened miscarriage. The authors concluded that, “For women with a subchorionic hematoma that is sonographically identified, fetal outcome is dependent on size of the hematoma, maternal age, and gestational age”. A metaanalysis by Tuuli et al. [39] which assessed trials in which the presence of a fetal heart was not identified, included 1,735 women with a subchorionic hematoma. 17.6 % of pregnancies progressed to miscarriage. Figure 4.3 shows a patient with a subchorionic hematoma from the author’s series.
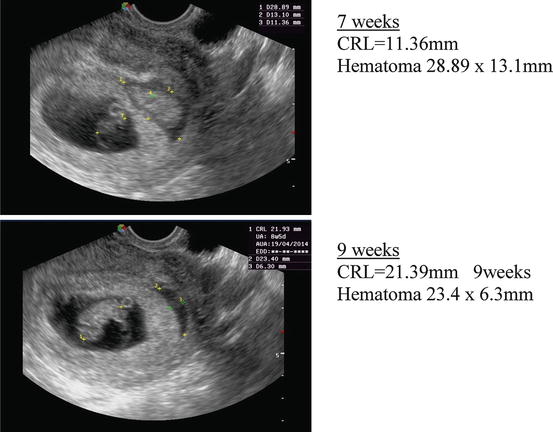

Fig. 4.3
Subchorionic hematoma at 7 and 9 weeks
There are two trials of progestogens in subchorionic hematoma. Both are open labelled observational studies. In the first study, Pelinescu-Onciul et al. [40] treated 125 women with micronized progesterone 600 mg/day. 18.7 % of pregnancies terminated in miscarriage. In the second study [41], 100 women, with threatened miscarriage and a viable embryo received dydrogesterone. There were 93 live births and 7 miscarriages. The difference in results was significantly better in the dydrogesterone group (RR = 2.04 CI = 1.05–3.97). However, these results should be treated with caution due to the methodological flaws of comparing two separate cohorts of patients who were not randomized.
5.2.2 Threatened Miscarriage After Recurrent Miscarriage
As stated above, vaginal bleeding occurs in 40–50 % of recurrently miscarrying women, even when the pregnancy is viable. Progestogen supplementation has been used in recurrent miscarriage with varying degrees of success, as described in the next chapter. As in threatened miscarriage, treatment can only affect a live embryo, or an embryo at a stage prior to 5.5 weeks (usually the earliest that a fetal heart can be detected.). A thorough search of the literature failed to find any reports of progestogen use to prevent threatened miscarriage developing to miscarriage in women with previous recurrent miscarriage. It is possible that progestogen supplementation prevents threatened miscarriage from occurring.
6 Safety and Side Effects
Safety and side effects are always a major worry when drugs are used in pregnancy. The major concerns about safety have centred on the effect of progestogens on the androgen receptor. The original progestogens were derivatives of 19 nor testosterone. These compounds led to varying degrees of masculinization of female fetuses. The masculinization included clitoral enlargement, and varying degrees of labial fusion. However, these side effects are not seen with other progestins. On the contrary, progesterone itself has anti-androgenic effects and has been reported to lead to hypospadias. In a case–control study, a relationship was reported between the use of progesterone and isolated hypospadias in two uncontrolled observational studies [42, 43]. Carmichael et al. [44], studied the risk of hypospadias and periconceptional progestin intake. Progestin-related hypospadia was reported by 42 (8.4 %) case mothers and 31 (2.4 %) control mothers, for intakes from 4 weeks before conception to 14 weeks of pregnancy. The crude odds ratio for progestin use at any time was 3.7 (95 % CI 2.3–6.0). Additionally, Rock et al. [45] reported one case of undescended testis and one case of meningomyelocele in 93 women treated with progesterone in the first trimester. Check et al. [46] found two cardiovascular malformations, omphalocele, hydrocephalus and club foot (with cleft palate) in 382 women exposed to either progesterone or 17-α hydroxy-progesterone. These studies had no control group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


