(1)
Foederatio Medicorum Helveticorum (F.M.H.), CH-3000, Bern, 15, Switzerland
(2)
Aerztekammer Nordrhein/Germany (A.E.K.N.O.), Tersteegenstr.9, D-40474 Düsseldorf/Golzheim, Germany
(3)
German Society of Anti-Aging Medicine e.V. (G.S.A.A.M.), Vallstedter Weg 114, D-38268 Lengede, Germany
(4)
Swiss Society for Anti Aging Medicine and Prevention (S.S.A.A.M.P.), Industriestrasse 3, CH-6345 Neuheim/Zug, Switzerland
An erratum to this chapter is available at http://dx.doi.org/10.1007/978-3-319-14385-9_15
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-3-319-14385-9_15
1 Introduction
Progesterone is the most basic of all steroid hormones. All other steroid hormones are produced physiologically by modifying the progesterone molecule into glucocorticoids, mineralocorticoids, estrogens and androgens. Progesterone acts by agonizing the progesterone receptor. It is known that numerous body organs have estrogen or androgen receptors, explaining the differences between both sexes. Sex differences are seen in behavior patterns, cyclic responses of the hypothalamus, hair production and distribution, in addition to the differences in the sex organs. Glucocorticoid receptors are also found in virtually all organs of the body. Therefore, it should be no surprise that progesterone receptors are widely distributed and that progesterone affects numerous organs in both sexes as well as the more well known effects of progesterone on the uterus and reproduction.
The discovery that men are also dependent on progesterone was exciting and piqued my interest. When a lack of progesterone is found in men, many conditions can be improved with progesterone supplementation. Two examples are prostate hyperplasia and erectile dysfunction. Indeed Lee [1] has reported the successful treatment of prostate cancer with progesterone and testosterone. In addition to progesterone being produced by the Leydig cells, it is also produced in the central nervous system by the glial cells of the brain and spinal cord [2] and in the Schwann cells [3] of peripheral nerves. Therefore, progesterone supplementation has been used successfully in depression, sleep disorders, multiple sclerosis, spinal cord neurodegeneration, brain trauma, CVA and epileptic seizures.
Both a gene defect or an age-related decrease may lead to a lack of progesterone.
Adequate substitution of progesterone for both prophylaxis and the correction of several deficiencies in the male and also in children and for a number of neurological disorders is not only justified, but highly desirable.
Since the late 1980’s a human identical form of progesterone has been available.
Micronization of progesterone has allowed the hormone to be administered orally rather than by deep intramuscular injection when dissolved in oil. As the objective of progesterone supplementation is to replace a lack of the physiological hormone, I only treat patients with bio-identical progesterone, and not synthetic progestogens.
However, as progesterone is not widely used outside of the accepted gynecological indications (described in other chapters in this book), appropriate control studies are urgently required.
I would like to dedicate this chapter to my patients, from whom I have learned what can be achieved when the lack of progesterone is corrected.
2 Progesterone in Males
Relatively little is known about normal values of hormones in men compared to the physiological levels in women. Although diminished levels of testosterone and DHEA are now often corrected with human identical hormones, the restoration of a healthy balance of hormones however, rarely achieves attention. As stated above the interaction of numerous hormones and enzymes is required in order to ensure an optimal hormone balance.
2.1 Biosynthesis of Sex Hormones
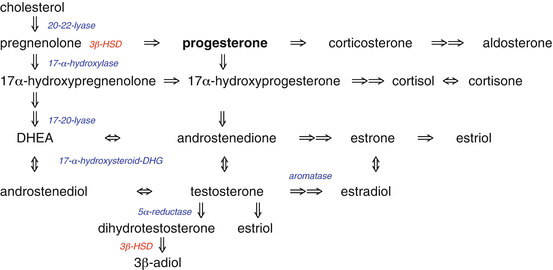
2.2 Progesterone Production in the Male
In men progesterone is produced in the Leydig cells of the testes, suprarenal gland, glial cells of the brain and the spinal cord [2] and in the Schwann cells [3] of peripheral nerves. Progesterone, Pregnenolone and their metabolites have been found in all of the above tissues. In the kidney, progesterone is metabolized to androgens.
2.3 Progesterone Receptors
The prostate is the male equivalent of the uterus and Skene’s glands. Since both develop from the distal part of the Müllerian duct and the surrounding glands, it is not surprising that the prostate has progesterone receptors as well as estrogen and testosterone receptors [4]. There are two isoforms of progesterone receptors: PR-A and PR-B. The PR-B isoform is a full-length receptor, the PR-A isoform has 164 amino acids fewer than the PR-B receptor. The ratio of PR-A and PR-B levels in the target cells determine the type and extent of the progesterone effect [5]. PR-A is responsible for progesterone-dependent reproduction and the PR-B for normal differentiating effects, e.g. the breast [6]. PR-B is more active than PR-A and is cell-specific. PR-A suppresses transcription activities of other steroid hormone receptors, including ERα and PR-B. Progesterone has similar effects as 3β-Adiol, the agonist of ERβ, which should rather be renamed the 3β-Adiol receptor, since 3β-Adiol is not an estrogen. Progesterone receptors have been identified in the heart, liver, sperm, epithelial cells of the eyes, brain and nerves and in the prostate. There are also two isoforms of 3β-hydroxysteroid dehydrogenase. Type I in the placenta and skin and Type II in the suprarenal gland, ovaries and testes. The progesterone receptor mediated suppression of gonadotropin reduces LH, FSH and thus testosterone in the male. It reduces pulsatile frequency and has little affinity for the androgen receptor [7].
The non-genomic effects of progesterone are regulated in men by intracellular receptors in the membrane. High concentrations influence the membrane liquid directly. Membrane-dependent progesterone effects are cell capacitation and LH receptor expression, leading to testosterone synthesis in the Leydig cells and interactions with the GABA receptor complex for sedation and anesthesia. Interactions also occur in fatty tissue and the kidneys.
2.4 Biological Activities of Progesterone
The highest levels of progesterone are found in the saliva of newborn infants. During the first month of life progesterone falls to one third of its previous level, and after 2 years the circadian rhythm has become established with high morning peaks and low evening values [8]. Progesterone displays a wide spectrum of biological activities in multiple tissues.
These effects can be stimulating or restraining, depending on the respective tissue, dose, point in time of application and progesterone receptor distribution.
2.5 Progesterone Prevents Prostate Cancer
Hereditary prostate cancer is associated with a defect in the 3β-hydroxysteroid dehydrogenase gene [9], which codes for the enzyme needed to metabolize progesterone from pregnenolone leading to a progesterone deficiency. The same enzyme is needed to metabolize 3β-Adiol from dihydrotestosterone (DHT). Hence the DHT level remains in excessive concentrations and 3β-Adiol, an important protective hormone, is reduced.
Additionally the age-related decrease of progesterone leads to a gradual decrease of testosterone and an increase of cell growth promoting estradiol [1, 9]. When hormone levels diminish in the aging male, markers of inflammation increase. These markers include C-reactive protein (CRP), interleukin-1β, interleukin-6, TNFα and total and free PSA levels and the quotient of both. In patients with prostate hyperplasia receiving progesterone, these parameters of inflammation decrease steadily back to the normal range. Micturition problems improve or even disappear with progesterone supplementation as the prostate shrinks. However, the process takes approximately 9–12 months.
Progesterone as the natural 5α-reductase inhibitor controls the metabolism of testosterone to DHT. The deficiency of 3β-hydroxysteroid-dehydrogenase (whether hereditary or age-related) leads to a decrease of progesterone, thus leaving DHT unopposed. Since 3β-Adiol cannot be metabolized from DHT, an excessive amount of DHT remains to promote prostate hyperplasia [10]. Progesterone is also the natural aromatase inhibitor controlling the effect of growth-promoting estradiol. The luminal cells of prostatic epithelia show high amounts of ERβ, whereas ERα is found primarily in the basal cells. In prostate cancer ERβ is down-regulated and ERα is spread to the luminal cells. ERβ is reduced 10 times and PR-A and PR-B are deficient, whereas ERα remains unchanged, leaving 17β-estradiol functions unopposed [11]. Theses mechanisms may explain why progesterone deficiency triggers the development of prostate cancer.
2.6 Other Important Effects of Progesterone in the Male
Progesterone is needed for fertility in men, since progesterone increases the volume of the ejaculate and improves sperm motility. The addition of progesterone to the Percoll medium in cases of assisted reproduction significantly increases sperm motility [12].
Progesterone improves sexual performance in rats [13]. Consequently, progesterone should also improve sexual performance in men due to similarities to human neuro-endocrine mechanisms. Indeed, patients receiving progesterone reported more frequent morning erections and distinctively improved sexual performance.
The anxiolytic effects of oral progesterone have been examined in a double-blind crossover study in 38 men. The Hamilton Anxiety Rating Scale was significantly reduced 4 h after progesterone administration and remained lower when examined after 9 and 24 h. Patients in the placebo arm of the trial only had a mild and non-significant reduction of the anxiety scale [14]. Additionally, the dose of antidepressants can be gradually reduced and often stopped completely [authors’s series, unpublished].
The sleep-inducing effects of progesterone are mediated by allopregnanolone and pregnanolone, the metabolites of progesterone [15]. Most of my patients report that sleep is improved with progesterone supplementation. Part of the mechanism whereby progesterone improves sleep patterns may be due to progesterone increasing pulmonary gas exchange and reducing alveolar CO 2 pressure, leading to improved respiration and therefore to undisturbed sleep [16, 17]. Under progesterone therapy, many patients snore less or stop entirely. Whether sleep apnoea is also due to progesterone deficiency requires further investigation.
Progesterone supplementation reduces cholesterol and returns the lipoprotein levels to normal. Normalization of cholesterol levels takes place especially quickly if in addition to progesterone supplementation, hypothyroidism is also corrected with a thyroid preparation (I personally use Novothyral, Merck Serono, Switzerland). After correction of the hormone profile, there are patients who could stop statins completely.
It has been reported that patients with asthma can stop their medication if substituted with progesterone [18]. I have only treated one patient with asthma, but the other medication could be stopped. However, trials are needed in asthma.
The anti-inflammatory effects of progesterone have been reported in men with active arthritis [19]. Intra-articular injection to one knee in 12 men produced a local anti-inflammatory effect, which lasted for 3 months. An especially impressive positive effect was observed in two men with polyarthritis, who did not respond to other therapies. The authors suggested either an immunosuppressive effect on lymphocytes or induction of immunosuppressive glycoproteins or binding of the glucocorticoid receptor, to explain the anti-inflammatory effect. Ginanneschi et al. [20] have treated 16 women with carpal tunnel syndrome by local injection of 17α-hydroxyprogesterone caproate. Progesterone therapy was compared to corticosteroids. Corticosteroid therapy was followed by a 1 month pain-free period. However, the pain-free period was six months after injection of the long acting progesterone derivative.
2.7 Standard Values of Progesterone in Men
At present, no laboratory has published normal values of progesterone in men. Lee used 5–8 mg progesterone transdermally and considered a level of 400 pg/ml in saliva as optimal [1]. Unfortunately, at present, no laboratory in Switzerland uses saliva levels to determine hormone levels. Rimkus [25] has used a progesterone level of 4–10 ng/ml in serum, and supplements progesterone levels if under 4 ng/ml. If the serum value increases to 10 ng/ml, it can be considered normal, but in my experience a progesterone level of 12 ng/ml is better. None of my patients had a normal value of progesterone. The progesterone levels are age-dependent lower with increasing age. Using a dose of 100 mg oral micronized progesterone (Utrogestan, Bessins International, Belgium) at night from day 6 of the month until the end of each month, (with a break from day 1–5 of the month, to prevent down-regulation of the receptor) [1] serum values of progesterone increased to 3.5 ng/ml up to 12 ng/ml. With substitution of DHEA or testosterone, the progesterone level increased to 6 ng/ml.
3 Progesterone, the Neurotrophic Hormone
Progesterone is not only produced in the Leydig cells of the testes, the ovary and adrenal cortex, but also in the glial cells in the brain and spinal cord [2] and in the Schwann cells of the peripheral nerves [3], (with especially high levels in the sciatic nerve). Consequently progesterone has many important effects on the brain and nerves. Some effects are described below.
3.1 Multiple Sclerosis (MS)
MS is an autoimmune inflammatory disease affecting the central nervous system, with demyelinization and neurodegeneration. The condition is characterized by relapses and remissions. Progesterone increases the number of oligodendrocytes [26] in the brain. Oligodendrocytes produce the protein myelin. Optimal levels of progesterone or pregnenolone lead to a statistically significant increase of myelin sheaths [27]. Progesterone suppresses matrix metalloproteinases [28], which maintain the inflammatory plaques in multiple sclerosis. Consequently in pregnancy under high progesterone levels exacerbation of MS does not occur. Post partum relapses are considered to be induced by the decreased levels of these steroids [29]. Progesterone has been shown to reduce the inflammatory reactions commonly seen in MS. This is due to the direct effect of progesterone on astrocytes and microglia [30]. I have one female patient, aged 62 with MS, who has received progesterone supplementation (200 mg micronized progesterone from day 6 until the end of the month) for the last 2 years. She has had improvement of her symptoms and can now use stairs more easily. Trials are urgently needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


