Fig. 8.1
The contraceptive toolbox
1.2.1 Derivatives of Testosterone
The basic molecule is testosterone. Due to elimination at the C 19 position the molecule becomes more progestogenic and the different variations are called C 19 Nortestosterone derivatives (see Fig. 8.2). The structure closest to testosterone can be seen in Norenthynodrel, Norethindrone, Norethisterone Acetate, Lynestrenol (Estranes). Further modification has lead to from the original estranes to gonanes like Levonorgestrel, Gestoden, Desogestrel, Norgestimate.

Fig. 8.2
C 19 Nortestosterone derivatives
A special molecule in this context is Dienogest.
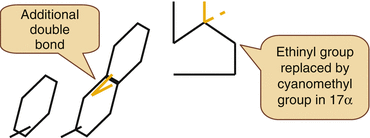
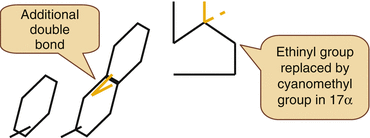
Dienogest is a 19 Nortestosterone derivative with a Cyanomethyl group instead of the usual Ethinyl Group in the 17 alpha position.
1.2.2 Derivatives of Progesterone
Subgroups can be distinguished depending on the different positions of double bindings between C atoms and the type of C group added.
An important molecule is Medroxyprogesteronacetate.

Another important progesterone derivatives in COCs are Cyproterone Acetate and Chlormadinone Acetate.


Both have a C-21 structure and are basically different from Testosterone derivates in their action in the body (see below). Both orally active progestogens suitable for use in combined hormonal contraceptives.
Another subgroup of progesterone derivatives are the 19 Norpregnanes.
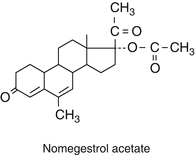



Their structure is very similar to progesterone. At position 19 the C methyl group is removed.
They have strong progestogenic activity. Nomegestrol acetate is orally active.
1.2.3 Derivatives of Spironolactone
Drospirenone is the only progestogen which is derived from spironolactone, which is known to counteract sodium retention and has an antiandrogenic action on the skin.
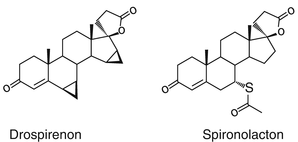

The drospirenone Molecule is a “mixture” of the progesterone and the spironolactone molecule.
1.3 Classification According to Interaction with Steroid Receptors
Depending on structure progestogens have different interactions with the various steroid receptors in the body. Steroid receptors are located on the membrane of target cells and are linked to the DNA/RNA and Protein production via different messenger systems. Two properties can be distinguished:
(a)
The binding capacity, i.e., the intensity with which the ligand of the molecule binds to appropriate receptor sites.
(b)
The direction and the intensity of the induced action. The steroid can bind to the receptor but does not induce any activity. The final effect is a reduction of the activity mediated by the receptor by competitive inhibition (the steroid binds to the receptor and thus blocks the binding site for a steroid that would activate receptor activity).
The receptors to which progestogens bind can be the following:
Progestogen receptor: The most important receptor to induce the desired effect.
Androgen receptor: Activation of androgen receptors mediate androgenic effects on hair growth and activity of the sebaceous glands. Some progestogens bind to this receptor and can either block or activate it (antiandrogenic properties see below).
Estrogen receptor: This receptor mediates effects in many tissues especially in the endometrial cells.
Glucocorticoid receptor: The glucocorticoid effect is linked to the activation of the coagulation system.
Mineralocorticoid receptor: This receptor mediates sodium retention.
Based on this classification of receptor activities several groups of progestogens can be differentiated. The clinical consequences of these differences are still controversial and research is going on.
Androgenic progestogens: Norethyodrel, Levnorgestrel and Norgestimate have androgenic properties. LNG seems to have not only an androgenic but also an antiestrogenic effect. Antiandrogenic progestogens: The most important compounds are the progesterone derivatives CPA and CMA and drospirenone. These molecules bind to the receptor and exert competitive inhibition.
Mildly antiandorgenic or neutral progestogens: GEST, DES, mainly interact with the progestogene receptor alone. Dienogest has a weak antiandrogenic action.
Antimineralocorticoid progestogens: Only Drospirenone has antimineralocorticoid action.
The different receptor effects can be described are shown in Table 8.1.
Table 8.1
Contraceptive progestogens and extra-progestogenic effects (adapted from Benagiano et al. [24]
Glucocorticoid activity | Estrogenic activity | Antiestrogenic activity | Androgenic activity | Antiandrogenic activity | Mineralocorticoid activity |
|---|---|---|---|---|---|
Medroxy progesterone acetate | Norethisterone acetate | Levonorgestrel | Levonorgestrel | Cyproterone acetate | Drospirenone |
Megestrol acetate | Desogestrel | Chlormadinone acetate | |||
Gestodene | Dienogest |
2 Health Risks of Combined Hormonal Contraceptives in Relation to the Progestogen
2.1 Third Generation
Several registry based studies published in the British Medical Journal (BMJ), particularly the Danish registry indicated that there might be an increased risk of venous thromboembolism (VTE) associated with the intake of 3rd and 4th generation COCs compared to preparations containing the progestogen levonorgestrel (LNG) or other first and second generation progestogens [1–5].
The relative risk increase was approximately 2 and absolute attributable risk was given—dependent on the base prevalence rate—between 2 and 8 per 10,000 users [6]. These results contrast with those of published prospective cohort studies, sponsored by Bayer Health Care, at the request of health authorities for a large postmarketing survey, which did not find a differences between the various generations of progestogens The discrepancy led to intensive scientific discussion among epidemiologists about possible confounders and biases in the published studies [7, 8]. The present recommendation is to inform women about the controversial results and the suspected, but not confirmed risk of the newer preparations; Health care professionals are required to balance the risks and benefits of the different preparations in a process of shared decision making with the individual woman. Women should be advised to continue hormonal contraception, in order to avoid the previously observed rise in the number of artificial abortions following the “pill scare” in 1995 [9, 10].
The unresolved scientific debate about VTE has continued, and was given new impetus by another publication based on the Danish Registry indicating that treatment with the transdermal patch, the vaginal ring and the implant were also associated with an increased risk of VTE, whereas the LNG releasing intrauterine system (LNG-IUS) did not or even decreased the VTE risk compared to non users. In this publication the authors recommended and gave practical advice how to switch from the contraceptives mentioned to either a LNG containing pill, a LNG-IUS or a non hormonal method [11, 12].
These publications with warnings about the increased risk of 3rd and 4th generation contraceptives lead to an intensive debate among epidemiologists about the limitations of registry based observational studies in comparison to other study designs and about the clinical validity of these results [13–17].
In summary, most studies suggest that the third generation progestins, desogestrel and gestodene (but not norgestimate), may be associated with a higher risk of venous thromboembolism when compared with levonorgestrel, a second generation progestin. However, the absolute excess risk is small, and may be outweighed by the many benefits of OCs, including prevention of an unwanted pregnancy, and a reduction in ovarian and endometrial cancer risk.
2.2 Antiandrogens
There are some special concerns and considerations regarding progestogens with an antiandrogenic action.
Cyproterone acetate. In two studies, a higher risk of VTE was seen when compared with contraceptives containing levonorgestrel [18, 19]. In a report from the Danish National Registry the risk was not significantly different from LNG (absolute risk 4.2 and 3.1 per 10,000 woman-years for levonorgestrel and cyproterone acetate, respectively) [20].
Chlormadinone Acetate. There are no large epidemiological studies with CMA, but the post marketing studies especially in Germany and Austria did not show an increased risk for VTE in users. The European Authorities demand however more surveillance studies to be able to determine the cardiovascular risk of chlormadinone which has been shown to be effective in reducing acne and dysmenorrhea.
Drospirenone. Drospirenone, a progestin that also has antiandrogen and antimineralocorticoid properties, has been associated with a greater risk of VTE when compared with levonorgestrel in some, but not all studies (see also above).
Two observational studies have reported that oral contraceptives containing drospirenone were associated with an excess risk of venous thromboembolism (similar in magnitude to the third generation progestins) [1, 2]. Two previous large, prospective, surveillance studies of new users of drospirenone-containing oral contraceptives subsequently reported that the thromboembolism risk was no different from that for other OCs It has been estimated that 9,000 women would need to be treated with a drospirenone OC in order to see one additional case of venous thromboembolism [7, 8]. After the publication of the two surveillance studies, two additional case-control studies and a registry-based cohort study reported a two- to threefold increased risk of VTE with OCs containing drospirenone compared with levonorgestrel [3–5].
An FDA sponsored study published after the Drug Safety Communication utilized computerized data files from two integrated medical care programs and two state Medicaid programs to obtain data regarding the risk of several cardiovascular endpoints in combined hormonal contraceptives users. The authors identified a final cohort that included 189,210 person-years of exposure to drospirenone. In adjusted analyses, drospirenone use was associated with a significantly higher risk of VTE relative to low-estrogen comparators (RR 1.74; 95 % CI 1.42–2.14) [21].
In 2012, based upon available data, the US Food and Drug Administration (FDA) added revised labeling to all oral contraceptives containing drospirenone, stating that they may be associated with up to a threefold higher risk of VTE compared with OCs with levonorgestrel and some other progestins [22]. The FDA does not advise women to stop drospirenone-containing OCs, but does suggest that an individual’s risk of VTE be assessed before starting one in a new OC user, or before considering using one in a woman who has been on an OC not containing drospirenone. Lastly, the warning notes that the VTE risk with drospirenone is small and still lower than the risk of VTE during pregnancy.
In 2011, the European Medicines Agency also concluded that drospirenone-containing birth control pills carry a higher risk of venous thromboembolism, but noted the overall risk of blood clot from any birth control method remains small and stopped short of advising women to stop taking pills containing drospirenone [23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


