Since the 1990s, progesterone has been studied for its possible role in preterm birth. Keirse in his meta-analysis of placebo-controlled trials, investigated the possible prophylactic use of 17 OHP-C in women at a high risk of pre-term birth. This analysis no effect in miscarriage, but did demonstrate a reduction in the incidence of preterm birth and the incidence of low birthwheight babies [21].
3 Key Differences in the Route of Administration
Although the pharmacokinetics and pharmacodynamics of progesterone have been well studied since 1935 when progesterone was first synthesized, its use in the pathophysiology of pregnancy remains controversial. One of these concerns is the optimal route of administration [22]. The rate of absorption is dependent on which pharmaceutical form is used, the blood flow at the site of administration and the solubility in the tissues into which the drug is administered. Progesterone can be administered by many different routes, but the main are oral, vaginal and intramuscular (Table 6.1).
Table 6.1
Routes of administration of progesterone
Different routes of administration | |
|---|---|
Different pharmacokinetics and dynamics | |
Intramuscular Supraphysiological plasma concentrations | |
Oral • Rapid increase n plasma concentration followed by a gradual decrease • First liver pass effect with several biological active metabolites • Specific activity on different target organs (uterus, brain…) | Metabolism – In the gut (bacteria with 5b-reductase activity) – In the intestinal wall (5a-reductase activity) – In the liver (5b-reductase, 3a- and 20a-hydrodylase activities) |
Vaginal • Stable plasma concentrations and consistent tissue levels • First uterine pass effect with targeted delivery into the endometrium • Minimal systemic effects | Metabolism – Normal vaginal bacteria and mucosa seem devoid 5a- and 5b-reductases – After vaginal, only a small increase in 5a-pregnanolone observed and 5b-pregnanolone levels were not affected |
3.1 Oral Administration
Oral administration is associated with better patient compliance, but has several disadvantages, the main disadvantage being extreme variability in plasma concentrations due to individual variability in gastric filling and enteropathic circulation. Food may influence the rate and extent of drug absorption by reducing the rate of gastric emptying, decreasing gastrointestinal motility, increasing gastrointestinal secretions and increasing splanchnic blood flow. Oral administration has another major shortcoming: after absorption, progesterone is first passed to the liver by the portal vein. The metabolites produced by the liver interfere with progesterone action; as shown by the discrepancy between progesterone levels and endometrial histology [23].
A number of synthetic oral progestogens have been developed to overcome progesterone’s low oral bioavailability. Their pharmacologic effects, however, differ from progesterone itself, and may result in increased androgenic effects, fluid retention, alterations in high density lipoproteins, headaches, mood disturbances, and possible teratogenicity dependant on the individual progestogen. Dydrogesterone however, seems to have no androgenic or anti-androgenic effect (See Table 2 in Chap. 2). Oral progesterone may also have other side effects such as nausea, headache and sleepiness. In an attempt to improve the characteristics of oral administration, micronized progesterone has been introduced. It is manufactured from chemicals derived from plants (Mexican wild yams, Dioscorea barbasco), yet it has a molecular structure identical to human progesterone [24]. Micronization of progesterone into particle sizes of <10 μm increases the available surface area, enhances the aqueous dissolution rate and intestinal absorption of progesterone. Suspension in oil and packaging in a gelatin capsule has been shown to further enhance the intestinal absorption of micronized progesterone [25].
3.2 Vaginal Administration
The vaginal route results in higher concentrations in the uterus [17, 22]. Bulletti et al. [26] tried to verify the hypothesis of a “first uterine pass effect,” suggested by the evidence of higher than expected uterine tissue concentrations after vaginal administration of progesterone. Three different hypotheses have been reported to explain the first uterine pass: direct diffusion through or between cells of the vagina to cells of the uterus, portal-like arrangements of lymphatics linking the upper vagina to the uterus, and a counter circulation system, much like a portal system, with vein to artery diffusion between the upper vagina and uterus. Bulletti et al. [26] demonstrated that a “first uterine pass effect” occurs when drug is delivered vaginally, confirming that the vaginal route permits targeted drug delivery to the uterus, maximizing the desired effects while minimizing the potential for adverse systemic effects (Fig. 6.2).
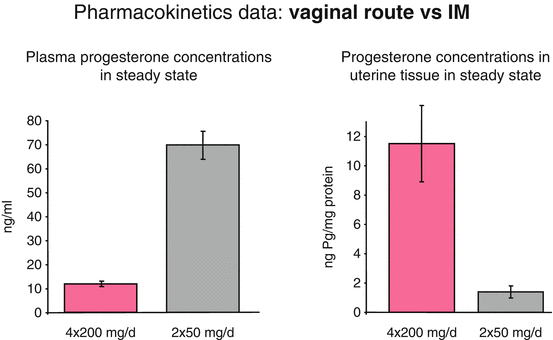

Fig. 6.2
Comparison between vaginal and intramuscular route of administration. Modified from Miles A et al. Fertil Steril 1994 [45]
After vaginal administration of progesterone, uterine tissue concentration have been found to exceed the levels achieved by systemic administration by more than tenfold; but the plasma levels are more than seven times higher after systemic administration compared to vaginal administration. The time to peak concentration is generally slightly less than after oral administration of micronized preparation. Moreover, after vaginal administration, plasma concentrations display a plateau-like profile, with a more constant concentration over time. Peak plasma levels of progesterone seem to be variable and not consistently greater or less than corresponding peak plasma values obtained after oral administration of micronized progesterone [25]. Cicinelli et al. demonstrated the direct transport of progesterone from vagina to uterus comparing progesterone concentrations in serum and endometrial tissue from hysterectomy specimens after vaginal or intramuscular administration of progesterone gel. They demonstrated that the ratios of endometrial to serum progesterone concentrations were markedly higher in women who received vaginal progesterone [27] (Fig. 6.3).
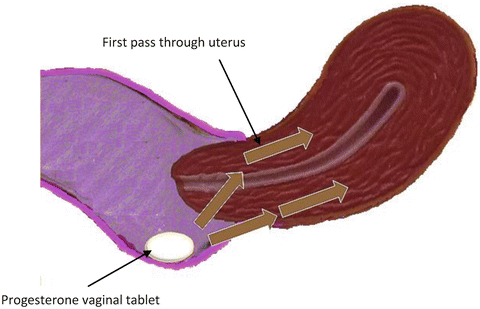

Fig. 6.3
Vaginal administration of progesterone: first uterine pass effect
3.3 Intramuscular Administration
Historically, the most common delivery method of progesterone has been intramuscular [25]. Progesterone administered intramuscularly can lead to local discomfort and extreme pain, and occasionally induces non-septic abscesses at the injection site. A large number of other side effects have been described including: hypersensitivity reactions, cough, dyspnea, tiredness, dizziness, genital itching, & increased risk of gestational diabetes, mood swings, headaches, bloating, abdominal pain, perineal pain, constipation, diarrhea, nausea, vomiting, joint pain, depression, decreased sex drive, nervousness, sleepiness, breast enlargement, breast pain, dysuria, polyuria, UTI, vaginal discharge, fever, flu-like symptoms, back pain, leg pain, sleep disorder, upper respiratory infection, asthma, acne and pruritus.
3.4 Other Routes of Administration
Other routes have been described. Progesterone can be administered by a transdermal patch as progesterone can penetrate the skin but it is rapidly metabolized by the 5-α-reductase enzyme, which converts it to 5-α-dihydro-progesterone, thereby lowering plasma progesterone levels. Rectal administration, is associated with variable absorption and there is insufficient scientific evidence concerning effects on the endometrium after rectal administration.
4 Progesterone and Preterm Birth
As stated above, PTB is the leading cause of perinatal mortality and morbidity. Its incidence has not declined (12 % of all birth) over the last 20 years, and, due to its long-term neuro-developmental sequelae, it is one of the major financial drains on health and educational resources [10, 28]. It is the major challenge in Obstetrics to-day to reduce the number of PTB’s or at least prolong pregnancy until fetal maturity is sufficient to ensure survival without excess morbidity.
The mechanism of human parturition is the expression of anatomic, biochemical, physiologic and clinical events that occur in the mother and in the fetus in both term and preterm labour. This pathway comprises: decidual/fetal membrane activation, increased uterine contractility and cervical ripening (dilatation and effacement). Preterm labor is the consequence of the pathologic activation of one or more of these elements. The apparent loss of progesterone sensitivity at term could be a consequence of several different mechanisms including: alterations in progesterone receptors (PR) isoform ratios, the anti-inflammatory function of progesterone, the catabolism of progesterone in the uterus into inactive compounds, changes in cofactor protein levels affecting PR transactivation and inflammation-induced trans-repression of PR [17].
Before undertaking any therapeutic strategy, careful identification is needed, so as to detect manageable conditions and fetal and/or maternal contraindication. The real challenge is to develop sensitive and specific tests that reliably detect these pregnancy changes before they became irreversible, and to find effective interventions capable of arresting the process of preterm labor and to enhance the effectiveness of current available interventions.
Regarding the management of threatened preterm labor, tocolysis and administration of corticosteroids to induce lung maturation are the first therapeutic tools; additionally bed rest and hydration are usually recommended in the management of these patients, although none have been shown to be clearly effective. Progesterone, other related synthetic compounds such as 17 OHP-C and other progestogens, have been tested in clinical trials for the prevention of preterm birth [29]. The three main groups of patients considered at particularly high risk include: patients with a previous preterm birth, patients with short cervix and patients with multiple pregnancy. Studies using synthetic progestogens to reduce the incidence of preterm delivery have been reported. However, there are mixed results. Progesterone itself however, has been demonstrated to be beneficial, especially in view of cost, availability and biological safety [30, 31].
The vast majority of clinical trials have been performed with various formulations of either microized progesterone or 17 OHP-C. The first has been administered through the vaginal route and the latter by weekly intramuscular injection. The use of both micronized progesterone and 17 OHP-C has been advised in asymptomatic women with a prior history of preterm birth as early prophylaxis for preterm, birth and in single pregnant, nulliparous women with asymptomatic cervical shortening (15 mm) detected with trans-vaginal ultrasound at midgestation. The tocolytic effect of oral micronized progesterone was first reported in a 1986 report by Erny et al. [32]. These investigators evaluated the effects of 400 mg of orally absorbed micronized progesterone or placebo in women at risk for premature labor; 88 % of the patients who were treated with oral micronized progesterone had decreased uterine activity compared with 42 % of the patients who received placebo. Furthermore Istwan et al. demonstrated that in patients with a history of one prior spontaneous preterm delivery prophylactic 17 OHP-C, confers a reduction in risk of preterm labour compared to untreated patients [33].
One of the main studies concerning the prophylactic use of vaginal progesterone to decrease the incidence of preterm birth in patients at a high risk, has been performed by Fonseca et al. [34]. Fonseca et al. demonstrated that the daily use of 100 mg vaginal progesterone is able to reduce the frequency of uterine contractions and the incidence of preterm birth. More recently it has also been demonstrated that the use of vaginal progesterone in asymptomatic women with a sonographic short cervix not only reduces the risk of preterm birth, but it is also able to reduce neonatal morbidity and mortality (Fig. 6.4, Table 6.2) [35]. In nulliparous women with a midtrimester cervical length <30 mm, the use of weekly intramuscular 17 OHP-C did not show a reduced incidence of preterm birth [36]. Comparing the use of vaginal progesterone with the intramuscular route of administration, a statistically significant decrease of the number of admission to the Neonatal Intensive Care Unit and a significant deficiency of severe side effects has been demonstrated [37].
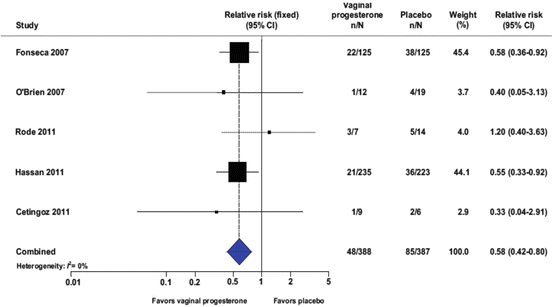

Fig. 6.4
Vaginal progesterone and reduction of preterm birth before 33 weeks of gestation: a metanalysis. From Romero R et al. Am J Obstet Gynecol 2012 [35]
Table 6.2
Vaginal progesterone in women with an aymptomatic short cervix in the midtrimester ultrasound decreases PTD and improves neonatal outcome
No. of events/total no. | ||||||
|---|---|---|---|---|---|---|
Outcome | No. of trials | Vaginal progesterone | Placebo | Pooled RR (95% CI) | I 2 (%) | NNT (95% CI) |
Preterm birth <37 wk | 5 | 144/388 | 165/387 | 0.89 (0.75–1.06) | 0 | – |
Preterm birth <36 wk | 5 | 108/388 | 136/387 | 0.82 (0.67–1.00) | 0 | – |
Preterm birth <35 wk | 5 | 79/388 | 118/387 | 0.69 (0.55–0.88) | 0 | 11 (7–27) |
Preterm birth <34 wk
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||