Fig. 11.1
Progesterone in endometrial cancer, chapter summary. MPA medroxyprogesterone acetate, MA megesterol acetate, LNG-IUD levonorgestrel releasing intrauterine device
2 Progesterone for Fertility Preservation
2.1 Are There Any Risks?
The development of endometrial cancer in young women usually results from a hyperestrogenic state that leads to endometrial hyperplasia. A tissue biopsy of atypical endometrial hyperplasia has been associated with a 29 % risk of progression to endometrial cancer [10], and endometrial cancers have been found in up to 43 % of patients with a preoperative diagnosis of atypical endometrial hyperplasia [11]. This high association warrants consideration in management decisions. According to a review of over 2,000 women aged 40 years or younger collected from the National Cancer Institute database, the majority of patients (75 %) had disease confined to the uterus, but approximately 17 % had stage III or IV disease [10]. These younger patients are also at increased risk of other pathological gynecologic conditions, including ovarian tumors. In a review of young women with endometrial cancer by Walsh et al. [11], 26 of 102 women (25 %) were found to have coexisting epithelial ovarian tumors (23 synchronous primaries and 3 metastases). Therefore, any decision to deviate from the standard approach of hysterectomy with oophorectomy and staging should take account of the risk of an undetected, and therefore subsequently untreated, synchronous or metastatic cancer. These studies confirm the need for thorough examination and careful patient selection, while highlighting the risks inherent in conservative management of an unstaged cancer.
Based on these data, the patient’s outcome may be adversely affected when choosing to pursue fertility preservation. In the absence of randomized trials, the largest study to evaluate this matter is a retrospective study by Koskas et al. [12] who examined 489 patients aged 40 or younger with grade 1 endometrial adenocarcinoma. The patients were divided into groups who underwent uterine preservation, ovarian preservation, or hysterectomy with oophorectomy. Ovarian and uterine preservation had no effect on either cancer-specific or overall survival. The limitations of Koskas et al’s. [12] study include the absence of information on which agents and treatment protocols were used and how they found no evidence for the 17–25 % of young patients with concomitant/metastatic adnexal carcinomas published in other reports [12, 13].
2.2 Workup Prior to Treatment
The optimal work-up to evaluate the extent of disease in young patients with endometrial cancer who desire to maintain their uterus has not been established. Every effort should be taken to ensure that the cancer is confined to the endometrium and low grade, and therefore likely to respond to hormonal therapy without compromising curability (Table 11.1). As a rule, the pretreatment evaluation should consist of a full workup for any signs or symptoms suspicious for advanced/metastatic disease (Tables 11.2 and 11.3).
I | Absence of frank myometrial invasion |
II | Well-differentiated (G1) endometrioid adenocarcinoma |
III | No contraindications for progesterone therapy |
IV | Potential for fertility |
V | Informed consent on the indications and limitations of progesterone therapy |
Table 11.2
Suggested procedures for the assessment of a patient with endometrial cancer seeking fertility sparing treatments
Procedure | Purpose |
|---|---|
Complete history and physical exam | Look for signs or symptoms suspicious for advanced/metastatic disease |
D&C | − Tumor grading − Possible therapeutic effect |
MRI | Assess myometrial invasion and loco-regional disease spread |
Diagnostic laparoscopy | Partial surgical staging |
Sentinel lymph node biopsy | Value to be determined |
Genetic counseling | Risk assessment for patient and family |
Table 11.3
Fertility sparing options; advantages and disadvantages
Drug | Dose | Advantages | Disadvantages |
|---|---|---|---|
Medroxyprogesterone acetate | 400–600 mg/day for at least 3 months | Well studied | Known side effects |
Megesterol acetate | 160–320 mg/day for at least 3 months | Well studied | − Known side effects − Might have higher recurrence rate compared to MPA |
Progesterone – intrauterine device | 20–65 mcg/day | Low systemic toxicity | − Limited data − Intra-uterine placement required |
Natural progesterone | 200 mg/days, days 14–25 | – | Limited data |
Hydroxyprogesterone | 500 mg/days | – | Limited data |
Norethisterone | 5 mg/days | – | Limited data |
Progestogens at various doses | – | – | Limited data |
2.2.1 Tissue Biopsy
Prior to initiating conservative management, dilatation and curettage (D&C) is recommended because it better defines the grade of the tumor compared to office endometrial biopsy [13]. Additionally, there might be value in the removal of most of the endometrial cancer cells by the D&C before starting hormonal treatment [14].
2.2.2 Imaging
Attempts should be made to rule out myometrial invasion, adnexal involvement and lymph node metastases, which are regarded as contra-indications for conservative management. MRI has proven to be superior to transvaginal ultrasound or CT for determining myometrial invasion [15]. Pooling of 11 studies, comparing T2-weighted imaging and contrast-enhanced magnetic resonance imaging, revealed similar positive predictive values for myometrial invasion of 0.65 and negative predictive values of 0.85 [16]. MRI is used to assess loco-regional disease spread [17], and Sironi et al. [15] reported a sensitivity and specificity of 74 % for MR assessment of superficial myometrial invasion, although the importance of superficial myometrial involvement on response to progestins is not clear.
2.2.3 Additional Invasive Procedures
There is an increased risk of concomitant adnexal involvement in premenopausal patients with endometrial cancer, reaching up to 25 % in the series from Cedars Sinai [11]. Consequently, some physicians perform a diagnostic laparoscopy at the time of D&C [18]. With the evolving data on sentinel lymph node biopsy for endometrial cancer, lymph node biopsy could be considered in selected cases [19].
2.2.4 Genetic Counseling
Women diagnosed with endometrial cancer at a young age are at increased risk for mismatch repair gene mutations associated with Lynch syndrome [20]. Hence, these women should also be referred for genetic counseling [21], as counseling might reveal important implications concerning the risk of adnexal pathology and colon cancer necessitating screening in these young patients and their families.
2.3 Prognostic Factors
Although the majority of carefully selected patients will respond to progestin therapy, there is at present no way to accurately predict who will respond.
Data remain scarce on clinical or pathologic predictors of response to progestin treatment in premenopausal women with complex and Grade 1 endometrial adenocarcinoma. Park et al. analyzed 148 patients (age ≤40 years) with stage IA, grade 1, endometrioid adenocarcinoma of the uterus who underwent fertility-sparing management using daily oral medroxyprogesterone acetate or megestrol acetate [24]. One hundred and fifteen (77.7 %) showed complete response to progestin treatment, and 35 (30.4 %) experienced recurrence after a median follow-up period of 66 months. A body mass index (BMI) ≥25 was the only significant factor associated with a failure to achieve cure (odds ratio [OR], 3.00; 95 % CI, 1.35–6.66; P = 0.007). A BMI ≥25 was also significantly associated with a higher risk of recurrence (OR, 2.14; 95 % CI, 1.06–4.31; P = 0.033). The use of MPA (compared to MA) (OR, 0.44; 95 % CI, 0.22–0.88; P = 0.021), continuing maintenance treatment (OR, 0.22; 95 % CI, 0.05–0.94; P = 0.042), and a previous pregnancy (OR, 0.25; 95 % CI, 0.11–0.56; P = 0.001) were significantly associated with a lower risk of recurrence [22]. Penner et al. [23] looked at the histopathologic features, using a qualitative abnormal endometrial architecture score, comparing pretreatment and follow-up endometrial specimens to identify predictors of resolution [25]. The score is composed of five features: polypoid, cribriform, papillary, budding and back to back endometrial glands. Resolution rates expressed as the Standardized Resolution Ratio (SRR) were highest in individuals with a low pre-treatment score and a BMI <35 (SRR = 1.48, p = 0.03), lower among subjects with a high pre-treatment score (SRR = 0.37, p < 0.03), and lowest in subjects whose first follow-up specimen showed persistent complexity, atypia, or carcinoma with adjacent stromal decidualization (SRR = 0.24, p = 0.002) [23]. The presence of progesterone receptors also predicts response to progestin therapy [24, 25]. In one study the response rate was 8 % (seven of 86 patients) for patients who were progesterone receptor-negative and 37 % (17 of 46) for patients who were progesterone receptor-positive (P < .001) [26]. In addition, PTEN and KRAS status in combination with the progesterone receptor expression in the tumor appear promising as biomarkers of response [25]. Further investigations in predictors of response may ultimately lead to personalized treatments for young women with endometrial cancer.
2.4 Types of Progesterone
At present, there is no consensus on the optimal medication, dose, or length of treatment. In a 2004 review, the most commonly used agents were medroxyprogesterone acetate 500–600 mg (MPA; 44 %) and megesterol acetate 160 mg (35 %) for at least 3 months [27]. Both regimens appear to have similar response rates although it has recently been suggested that the recurrence rate is higher after megestrol acetate compared to medroxyprogesterone acetate [22].
Additionally, treatment has been reported with the levonorgestrel intrauterine device (LNG-IUD) (MirenaTDM) that releases 20 mcg of levonorgestrel per day [28], in combination with hysteroscopic resection [29], medroxyprogesterone acetate [30] or GnRH analogues [31]. Other treatments used include intramuscular 17-hydroxyprogesterone, oral contraceptive pills, norethisterone, dihydrogesterone, and natural progesterone either utilized alone or in a combination of progestin agents [7, 32].
The choice of progestin should be based on measurable outcomes, including efficacy, side-effects, and patient tolerability. Orally administered progestins are not without side-effects, including mood alterations, headaches, weight gain, breast pain and/or tenderness, and increased risk of thrombus formation. Thrombosis is a serious adverse reaction to MPA. It is caused by the inhibitory activity of MPA against plasminogen activator [33]. Thrombosis can be fatal especially if leading to cerebral infarction, myocardial infarction, or pulmonary embolism. Clotting factors should be checked monthly, and treatment with MPA should be discontinued on detection of clotting abnormalities. A prospective trial using 600 mg MPA [34] reported that the most common side effects were weight gain and liver dysfunction. There were no cases of thromboembolism. Progesterone therapy is contraindicated in the presence of a history of thromboembolus, breast cancer, or hepatic dysfunction. The progesterone-releasing IUD might be a means of achieving a localized effect within the endometrium while avoiding the adverse systemic toxicity. There is no consensus regarding the optimal progestin duration. Progestin therapy has an impact on the endometrial cells as early as 10 weeks after initiation of treatment, but most physicians suggest the need for a minimum of 3 months of treatment before assessing the response with endometrial hyperplasia and even longer for endometrial cancer [34]. Obese and anovulatory women have been shown to require longer periods of progestin therapy to attain a complete response, and are more prone to relapse [22, 23].
2.5 Outcome
Although the first publication describing fertility preserving, conservative treatment with hormones was published in 1961 [35], the number of publications describing the outcome is still limited (Fig. 11.2) and many questions remain. The possibility of publication bias in the studies analyzed should be borne in mind. Studies showing treatment success are more likely to be reported and published than negative trials, leading to overestimating the success rate. In a recent metaanalysis [36], including 34 observational studies, the authors evaluated the regression, relapse, and live birth rates of 408 women diagnosed with early-stage endometrial cancer. The primary studies included the outcome of women with well-differentiated endometrial cancer with 386 women being classified as G1 and 22 women with moderate or poor differentiation (G2 or G3). Half of the studies were prospective cohorts (17 of 34) and only in 6 of the 34 studies, was the follow-up more than 5 years. Overall, resolution occurred in 76 % (301/408) of reported patients (Table 11.4), and 89 (40.6 %) responders relapsed during follow-up. 75 women achieved at least 1 live birth, yielding a live birth rate of 28 %.
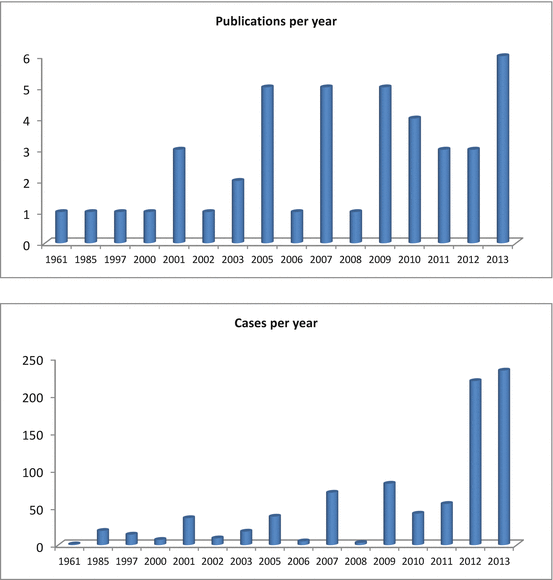

Fig. 11.2
Number of publications and number of reported cases treated conservatively
Table 11.4
Overview of studies and outcomes of progestogen treatment, adapted from Gallos et al. [36]
Study (year, reference) | Total of patients | Regressed (%) | Relapsed (%) | Live births (%) |
|---|---|---|---|---|
Bokhman (1983, [61]) | 19 | 15 (79) | – | – |
Randall (1999, [62]) | 14 | 10 (71) | 1/10 (10) | 3/14 (21) |
Kim (2000, [63]) | 7 | 4 (57) | 2/4 (50) | 0/7 (0) |
Imai (2001, [64]) | 14 | 8 (57) | 3/8 (38) | 2/14 (14) |
Kaku (2001, [65]) | 12 | 9 (75) | 2/9 (22) | 1/12 (8) |
Duska (2001, [2]) | 12 | 9 (75) | – | – |
Wang (2002, [66]) | 9 | 8 (89) | 4/8 (50) | 2/9 (22) |
Gotlieb (2003, [67]) | 13 | 13 (100) | 6/13 (46) | 3/13 (23) |
Jadoul (2003, [68]) | 5 | 3 (60) | 0/3 (0) | 3/5 (60) |
Niwa (2005, [69]) | 12 | 12 (100) | 8/12 (67) | – |
Ota (2005, [70]) | 12 | 5 (42) | 2/5 (40) | 2/12 (17) |
Yahata (2005, [71]) | 8 | 7 (88) | 7/7 (100) | 2/8 (25) |
Yang (2005, [72]) | 6 | 4 (67) | 2/4 (50) | 2/6 (33) |
Le Digabel (2006, [73]) | 5 | 3 (60) | 1/3 (33) | 0/5 (0) |
Elizur (2007, [74]) | 8 | 8 (100) | 3/8 (38) | 4/8 (50) |
Minaguchi (2007, [75]) | 18 | 14 (78) | 5/14 (36) | 1/18 (6) |
Ushijima (2007, [34]) | 22 | 14 (64) | 8/14 (57) | 3/22 (14) |
Wheeler (2007, [76]) | 21 | 7 (33) | 1/7 (14) | – |
Yamazawa (2007, [77]) | 9 | 7 (78) | 2/7 (29) | 3/9 (33) |
Li (2008, [78]) | 3 | 3 (100) | 0/3 (0) | – |
Eftekhar (2009, [79]) | 21 | 18 (86) | 3/18 (17) | 2/21 (10) |
Hahn (2009, [80]) | 35 | 22 (63) | 9/22 (41) | 8/35 (23) |
Han (2009, [81]) | 7 | 7 (100) | 0/7 (0) | 5/7 (71) |
Signorelli (2009, [82]) | 11 | 6 (55) | 4/6 (67) | 4/11 (36) |
Yu (2009, [83]) | 8 | 6 (75) | 1/7 (17) | 0/8 (0) |
Mao (2010, [84]) | 6 | 4 (67) | 0/4 (0) | 3/6 (50) |
Mazzon (2010, [85]) | 6 | 6 (100) | 0/6 (0) | 4/6 (67) |
Minig (2010, [31]) | 14 | 8 (57) | 2/8 (25) | 1/14 (7) |
Cade (2010, [7]) | 16 | 10 (63) | – | – |
Laurelli (2011, [29]) | 14 | 14 (100) | 1/14 (7) | 1/14 (7) |
Park (2011, [86]) | 14 | 13 (93) | 3/13 (23) | 13/14 (29) |
Perri (2011, [87]) | 27 | 24 (89) | 9/24 (38) | 12/27 (44) |
Total | 408 | 301 (76 %) | 89/267 (40.6 %) | 75/325 (28 %) |
3.6 % of patients were diagnosed with ovarian malignancy during follow-up. It is unclear whether these represent concurrent ovarian malignancies or metastatic ovarian involvement from the primary endometrial neoplasm. There were also ten women (1.8 %) diagnosed with stage II disease or greater following treatment failure, and there were two deaths reported (0.5 %). Another recent systemic review by Gunderson et al. [32] reported oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 endometrial cancer. Forty-five studies with 391 study subjects were identified including 280 women that had grade 1 endometrial adenocarcinoma. The median age for the overall cohort was 31.7 years (range 19–80 years). When stratified by disease type, the durable complete response rate was significantly higher in women with complex atypical hyperplasia (65.8 %) compared to those with carcinoma (48.2 %; p = .002). The rate of initial response in women with complex atypical hyperplasia was also significantly higher (85.6 %), than women with carcinoma (74.6 %; p = 0.03). Disease recurrence was more likely to occur in the carcinoma cohort (35.4 %) than the hyperplasia group (23.2 %; p = .03). Further, persistent disease was noted in only 14.4 % of women with complex atypical hyperplasia compared with 25.4 % of those with carcinoma (p = .02). Reproductive outcomes did not differ between the cohorts.
2.5.1 Repeat Treatment for Recurrence After Complete Response?
Park and colleagues recently published a retrospective multicenter study that shows the safe and effective outcome of re-treating 33 young patients who still wanted to preserve fertility following recurrence after a complete response to progestins [37]. Five of the 33 women failed to respond to a second conservative approach, and another five patients recurred after a second complete response. Three received a third cycle of progestins and two responded again. Five patients delivered six healthy babies following this second conservative approach. The responders were followed for a mean of period of 51 months, no patient died of disease or suffered an adverse outcome.
2.5.2 Outcome for Progestin Releasing Intrauterine Devices
Levonorgestrel releasing intrauterine devices (LNG-IUD) are associated with contraceptive efficacy, powerful reduction of menstrual blood volume through suppression of endometrial growth, and accompanying relief of menstrual pain [38]. It has also been shown that the use of LNG-IUD in combination with hormone replacement therapy during or after menopause can prevent endometrial cancer [39]. The efficacy of the LNG-IUD in suppressing the growth of endometrium has also been shown in patients with “non-atypical” endometrial hyperplasia, with a response seen in 96 % of all cases within 1 year and 92 % within 2 years after insertion [40]. Efficacy in patients with endometrial cancer is presently being investigated. Preliminary data obtained from two separate studies suggests that progestin treatment provided by an IUD in 22 patients with grade 1 Stage I endometrial cancer [29, 41] was followed by a 68 % (15/22) complete response after six months or longer compared to 72 % (73/102) of patients on oral progestin [8]. No relapses or progressions were reported after 6–71 months of follow-up. Fertility outcomes were not reported. In addition, a few more studies, all with a small number of patients [30, 31] suggests that treatment with oral or intrauterine progestin is similarly effective. Large prospective trials for LNG-IUD are presently underway in order to clarify some of the unresolved issues (Table 11.5).
Table 11.5




Progestin and endometrial cancer, ongoing clinical trials and time of expected results
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


