Fig. 9.1
Inflammatory reaction and neoangiogenesis around endometriotic lesion. U Uterus, US uteroscacral ligaments. These can be seen to be edematous and inflamed. E endometriotic lesion, BV dilated blood vessels
Endometriosis has many features of an autoimmune disease as there is increased polyclonal B-cell activity, high B-cell and T-cell counts, but with abnormal function [11, 12], and reduced natural-killer-cell activity [7, 12] High serum concentrations of IgG, IgA, and IgM autoantibodies [12, 13] and anti-endometrial antibodies have been reported [14]. Additionally, there is a high concordance of other autoimmune diseases or phenomena in women with endometriosis such as systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome, autoimmune thyroid disease, allergies, asthma and eczema [15]. Several studies have reported an association between autoimmune thyroid disease and endometriosis associated infertility, as shown by a high prevalence of positive anti-TPO antibodies [16].
Rather than being autoimmune in origin, the inflammatory reaction may be a response to the invasive properties of ectopic endomerium. Ectopic endometrial tissue fragments can attach to and invade the peritoneal surface. Matrix metalloproteinases (MMP) degrade extracellular matrix. MMP 7 and MMP 11 are normally expressed in the endometrium during menstrual breakdown and subsequent oestrogen-mediated endometrial proliferation. MMP 7 and MMP 11 are normally suppressed by progesterone during the secretory phase [17]. However, the ectopic endometrium is resistant to the effects of progesterone action due to non expression of the enzyme 17β hydroxysteroid dehydrogenase in women with endometriosis. Persistent expression of MMP might enable endometrial tissue to invade the peritoneal surface.
Survival of ectopic endometrial tissue in the peritoneal cavity is crucial for the establishment of viable implants. Downregulation of proapoptotic genes and upregulation of antiapoptotic genes of the BCL2 and BAX families, have been reported endometriotic lesions [18]. Hence, there may be an intrinsic abnormality in endometriosis that permits ectopic endometrium to attach, survive, invade, and establish a blood supply.
Nuclear factor kappa B (NF-kB) may be crucial for mediating several biochemical processes associated with endometriosis [19]. NF-kB is activated by proinflammatory cytokines and oxidative stress and is increased in endometriotic lesions. NF-kB activation leads to the expression of a number of genes involved in inflammation, such as IL-1, IL-6, IL-8, and cyclooxygenase-2 [20]. Endometriotic tissue has been shown to activate NF-kB [21]. By activating proinflammatory genes, NF-kB perpetuates inflammation and macrophage recruitment. In addition to the inflammatory cascade, NF-kB regulates genes involved in antiapoptosis, tissue invasion, cell proliferation, and angiogenesis. In healthy women, NF-kB-DNA binding is decreased in the secretory phase relative to the proliferative phase, which may be due to the anti-inflammatory action of progesterone [21] . However, in women with endometriosis, NF-kB-DNA binding remains elevated during the secretory phase [22].
2.1 Effect of Sex Hormones on Inflammatory Reaction
Hormonal alterations may influence the ability of endometriotic cells to proliferate, attach to the mesothelium and evade immune mediated clearance [23]. Figure 9.2 shows the effect of various hormones and medications on the development of endometriosis. Endometriotic implants have increased expression of aromatase. and decreased expression of 17β-hydroxysteroid dehydrogenase [24] The consequence is a marked increase in the locally bioavailable estradiol concentration. Estradiol stimulates the production of prostaglandin E2 which further stimulates aromatase activity [25]. In addition to estrogen dependence and biosynthesis, there is progesterone resistance in the pathophysiology of endometriosis [26]. Endometriotic lesions have reduced progesterone receptor expression when compared to eutopic endometrium, and an absence of progesterone receptor-B [27]. It has been reported that Akt (protein kinase) may be overactive, thus promoting progestogen resistance, and promotes the survival and proliferation of the diseased cells [28]. Increased Akt may be sufficient to downregulate progesterone receptor protein expression.
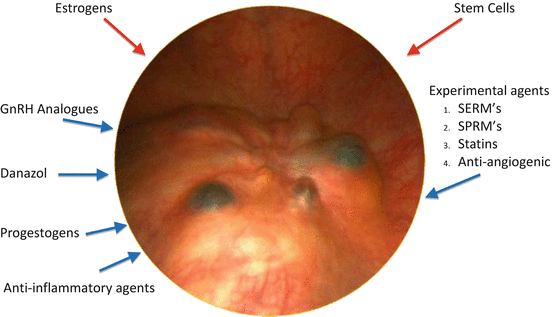

Fig. 9.2
Medications acting on endometriosis. Red arrows = stimulate endometriosis. Blue arrows indicate agents inhibiting endometriosis. SERM’s = selective estrogen receptor modulators, SPRMS = selective progesterone receptor modulators
HOX genes, are dynamically expressed in the endometrium, where they are necessary for endometrial growth, differentiation, and implantation. In the human endometrium, the expression of HOXA10 and HOXA11 has peak expression at implantation in response to rising progesterone levels. However, maximal HOXA10 and HOXA11 expression does not occur in endometriosis, due to altered progesterone receptor expression or a dysregulated progesterone response. Consequently, other mediators of endometrial receptivity that are regulated by HOX genes, such as pinopodes, αvβ3 integrin, and IGFBP-1, are downregulated in endometriosis. HOXA10 hypermethylation may silence HOXA10 gene expression and account for decreased HOXA10 in the endometrium of women with endometriosis. Silencing of progesterone target genes by methylation is an epigenetic mechanism that mediates progesterone resistance. The relatively permanent nature of methylation may explain the widespread failure of treatments for endometriosis-related infertility [29].
Normal apoptosis mechanisms are suppressed in endometrial cells from women with endometriosis and within endometriotic lesions. Increased estrogen availability due to local estradiol synthesis and increased estrogen sensitivity lead to exaggerated protein kinase (Akt) activation and apoptosis inhibition [30].
2.2 Innervation of Endometriosis
In endometriosis there appears to be neoneurogenesis. The functional layer of eutopic endometrium (functionalis and basalis) is highly innervated in endometriosis [31] as are the ectopic lesions [32]. Small nerve fiber density assessed in endometrial biopsies from women with early stage endometriosis was 14 times higher than in biopsies from healthy controls [33]. Pain severity scores correlated with the presence of nerve fibers in both peritoneal and rectovaginal endometriotic lesions [34]. Persistent stimulation of nascent nociceptors in endometriotic lesions by inflammatory mediators may lead to central sensitization and neuropathic pain [35].
3 Evasion of Immune Clearance
Normally, endometrial cells which have been shed into the peritoneum are cleared by the immune system. Failure of the clearance mechanism may predispose to the implantation and growth of endometriosis. Endometrial cells have been found to be resistant to lysis by natural killer (NK) cells when compared to the endometrium from women without disease [7]. Shedding of intercellular adhesion molecule-1 (ICAM-1) by endometrial stromal cells from women with endometriosis may be one mechanism whereby these cells escape NK mediated clearance [36]. Impaired NK cell function (by downregulation of the NK1receptor and compromised macrophage function in endometriosis may further contribute to decreased clearance of lesions.
4 Treatment
No treatment of endomeriosis is curative, but based on tiding the patient over with pain relief, or optimising the possibility of fertility. The principles of treatment are summarised in Table 9.1 and include:- 1. Debulking and restoration of anatomy, by surgery. 2. Reduction of the estrogen required to maintain endometriosis. This can be achieved with GnRH analogs or Danazol 3. Reduction of the inflammatory response. This approach uses progestogens or anti-inflammatory agents. The object of medical management is to control pain and prevent recurrence of endometriosis. Medical treatment can be used on its own or as an adjunct to surgery.
Table 9.1
Management of endometriosis: therapeutic intervention
Debulking | Surgery |
|---|---|
Estrogen reduction | 1. Danazol 2. GnRH analogues 3. GnRH antagonists |
Reducing inflammatory response | 1. Progestogens 2. Oral contraceptive pills 3. Anti-inflammatory agents |
Endometrial atrophy | 1. LNG-IUS 2. Gestrinone |
4.1 Surgical Treatment
Surgical excision or ablation and adhesiolysis is an effective initial approach to treatment, whether for pain relief, or to enhance fertility [37]. There is widespread consensus that lesions should be excised where possible, especially deep endometriotic lesions [38]. Moreover, the surgery may be complex, particularly if other organs are involved such as bowel bladder or ureter. Moreover, even after excision which seems to be complete, there is often recurrence. Recurrence has been reported to vary from 10 to 55 % within 12 months [39], with recurrence affecting approximately 10 % of the remaining women each additional year [40]. The necessity for repeat surgery is higher in women younger than 30 years at the time of surgery [41]. Initial surgery produces better results than subsequent surgical procedures [42].
When there is deep infiltrating endometriosis, there is a dilemma. Incomplete resection may reduce symptomatic outcomes [43], but very extensive surgery increases the risk of ureteric and rectal injuries etc. [44]. Hence, it is clear is that surgical experience and expertise are required for endometriosis surgery, and that surgery should preferably be undertaken in centers of expertise.
Observational studies have suggested improved pain outcomes for women who undergo hysterectomy for Stage IV endometriosis [45]. However, hysterectomy is obviously reserved for women who do not require fertility. 96.9 % of women become asymptomatic after menopause [46]. It should also be borne in mind that as long as the patient has endogenous estrogen production, surgery is not curative, and in most cases only cytoreductive.
4.2 Progestogens
4.2.1 Mode of Action
Progestogens have been used to control the pain of endometriosis for many years. Progestogens are effective at a number of different levels. Some progestogens have anti-gonadotropic actions effect which inhibits ovarian function to create a hypoestrogenic environment. It was assumed that by directly acting on endometrial progesterone receptors progestogens may induce decidualization and atrophy of endometriosis. Progestogens can also reduce the inflammatory response. Hence, progestogens are frequently used as first-line therapy for the treatment of endometriosis. Progestogens do not reduce estradiol levels as much as GnRH agonists. Progestogens are advantageous over GnRH analogs as progestogens do not induce a medical menopause and are not associated with hot flushes and other hypo-estrogenic side effects such as decreased bone mineral density.
The concept of progestogens acting on progesterone receptors and inducing decidualization as in normal endometrium does not seem to be valid. Endometriotic foci contain very few progesterone receptors [47]. The enzyme 17β hydroxysteroid dehydrogenase is absent in endometriotic tissue [48] and cannot be activated by progestogens. Progestogens also reduce the synthesis of their own receptors, which down regulates the sensitivity of the implants during long-term treatment.
Progestogens most probably acts by reducing the inflammatory response. TNF-α and estradiol induce the proliferation of endometriotic stroma cells via NF-kB, whereas progestogens reduce TNF-α induced NF-kB activation [49]. Progesterone itself is associated with decreased IFN-γ & increased IL-10 in endocervical fluid [50], upregulation of LIF mRNA expression in vitro. [51], and inhibits NK cell activity. The synthetic progestogen dydrogesterone has also been shown to modulate immune responses via suppression of IL-8 production in lymphocytes, inhibition of IFN-γ and increasing levels of IL-4 [52]. The increase in nitric oxide production seen with dydrogesterone may also play an important anti-inflammatory role [53].
4.2.2 Different Progestogens
Many progestogens have been have been used for the treatment of endometriosis. These include medroxyprogesterone acetate (administered by intramuscular injection), desogestrel, dienogest, cyproterone acetate, dydrogesterone etc. administered orally, either alone, or in combination with oestrogens in the combined oral contraceptive pill or levonorgestrel absorbed from an intrauterine contraceptive device. Progestogens have many beneficial effects. However, the results do not differ very much from the use of the combined estrogen progestin oral contraceptive pill. Some specific progestogens are described below.
Dydrogesterone
Dydrogesterone is a stereoisomer of progesterone manufactured by treating progesterone with ultraviolet light. Dydrogesterone stimulates the progesterone receptor directly without affecting progesterone levels. Dydrogesterone also binds the receptor 50 % more than progesterone itself [54]. Dydrogesterone does not stimulate the androgen, glucocorticoid or estrogen receptor.
There are numerous small comparative studies and case reports of dydrogesterone in endometriosis since the 1960s. Schweppe [55] summarized seven control studies of dydrogesterone. Doses between 10 and 60 mg/day, were used for various numbers of days per cycle, and over periods of 3–9 months. The majority of women became symptom-free or experienced a significant reduction in the number/severity of symptoms. These findings have been supported by laparoscopic examination in several of the studies. There was an overall success rate of approximately 70–90 %. Laparoscopic examination in several of the studies supported these findings. However, a Cochrane review published in 2012 [56] which included 13 randomised controlled trials evaluating the use of progestogens, included found only one RCT for dydrogesterone [57]. Sixty-two women were randomized to dydrogesterone or placebo. Only 39 women completed the study and underwent a second look laparoscopy. There was no significant improvement in objective efficacy at 6 months compared to placebo (OR 0.53, 95 % CI 0.14–1.94) Nor were there any differences observed in the change in pain score at 12 months of follow up (OR 0.80, 95 % CI 0.27–2.37; NS). However, the wide confidence intervals and small number of patients indicates that Schweppe’s [55] figures are probably more relevant.
Cyclic application of dydrogesterone has also been shown to induce regular menstruation with reduced blood loss and fewer days of bleeding, combined with excellent symptomatic relief, in women suffering from dysmenorrhea. Dydrogesterone is especially useful in patients desiring pregnancy Because dydrogesterone does not inhibit ovulation it can be used for symptomatic treatment of pain, reduction of bleeding problems and cycle control.
Dienogest
Dienogest is a synthetic orally active progestogen. As dydrogesterone, dienogest is highly selective for the progesterone receptor and exerts a strong progestational effect. However, dienogest differs from dydrogesterone in that it has a moderate antagonist action on the androgen receptor, and has a moderate antigonadotropic effect [58]. However, there is no androgenic, glucocorticoid, mineralocorticoid activity or estrogenic activity. The therapeutic dose (2 mg) inhibits ovulation in healthy women with normal menstrual cycles [59]. However, dienogest only moderately reduces oestrogen levels, hence, dienogest does affect bone mineral density [60]. Dienogest does not reduce sex hormone-binding globulin, is bound unspecifically to albumin and does not accumulate using oral doses of 2 mg/day [61].
Two prospective placebo controlled randomized studies assessed dienogest 2 mg daily against placebo [58] or versus leuprorelin depot [60]. Both trials showed a significant improvement in endometriosis-related symptoms, and a similar effectiveness to GnRH agonist therapy. However, the bleeding pattern differed substantially between the two groups. In the leuprolide group most women had infrequent bleeding in the first 90 days and amenorrhea after prolonged treatment. In the dienogest group prolonged and irregular bleeding were frequent in the first 90 days of treatment. Bleeding problems occurred in up to 80 % of patients within the first 3 months of treatment.
In a single-arm extension study of treatment for 15 months and follow-up 6 months after discontinuation of treatment, dienogest was shown to reduce pain symptoms with normalisation and long term relief of symptoms even after treatment discontinuation.
Dienogest has a good safety and efficacy profile, with good tolerability, antiandrogenic action and weak antigonadotropic activity, combined with typical characteristics of 19-norprogestins: strong suppressive action on the endometrium in low doses, a short half-life and high bioavailability [62]. However, there are no trials comparing the efficacy and tolerability of dienogest to OCPs or other progestogens.
Medroxy Progesterone Acetate (MPA)
MPA can be administered orally in a dose of 15–50 mg orally or injected as a depot form (DMPA). Bergqvist et al. [63] compared MPA to placebo. There was a greater quality of life after MPA and pain relief. Telimaa et al. [64] reported the results of a prospective, randomized trial comparing MPA to Danazol. A 50 % regression rate of ectopic implants and 13 % partial regression with scar formation was reported in the treatment group compared to 12 and 6 %, respectively, in the placebo group, and a net reduction in pain symptoms after treatment compared to placebo. (OR = 0.70, CI −8.61 to −5.39; P < 0.00001). When Danazol and MPA were compared, both alleviated endometriosis-associated pelvic pain, lower back pain and defecation pain, but they did not differ from each other in these actions. The authors concluded that because of good efficacy and tolerance, high-dose MPA is a useful alternative in the hormonal treatment of endometriosis. However, MPA has glucocorticoid and androgenic effects. Brown et al. [56], have reported significantly more cases of acne (six versus one) and oedema (11 versus one) in the medroxyprogesterone acetate group compared with placebo. The dose is 20 mg to 100 mg daily. Harrison and Barry-Kinsella [65] published the results of a placebo controlled trial. Initial and second-look laparoscopy were performed to grade the lesions according to the revised American Fertility Society stages. Surprisingly, both MPA and placebo therapy achieved similar statistically significant reductions in stages and scores at second-look laparoscopy. However, MPA was more effective in improving overall well-being. The authors concluded, that as both MPA and placebo were equally effective in treating endometriosis over a 3-month period, and questioned the role of using MPA altogether.
MPA can also be administered intramuscularly in a depot form (DPMA). DPMA is long acting, and a 150 mg. dose may only need to be repeated after 3 months. Vercellini [66] compared 150 mg of depot medroxyprogesterone (DMPA) every 3 months with a 20 μg oral contraceptive pill (OCP) with 50 mg danazol. Both the pill and danazol were taken for 3 weeks out of 4. The primary endpoint was the degree of satisfaction at the end of therapy. Pain reduction with MPA was as effective as danazol. DPMA has also been compared to leuprolide [67]. Symptoms of dysmenorrhoea were significantly reduced in the DMPA group at 6 months compared with leuprolide (OR 0.19, 95 % CI 0.05–0.69; P = 0.01) but this effect was short lived, and not present at the 12 months follow-up (OR 0.63, 95 % CI 0.37–1.08). There were no differences between groups at 12 months follow up, regarding dyspareunia after 6 months. At 12 months fewer women in the leuprolide group reported dyspareunia (OR 4.83, 95 % CI 2.14–10.93).
Side effects include breakthrough bleeding in approximately 40 % of patients, nausea, breast tenderness, fluid retention and depression.
Cyproterone Acetate
Vercellini [68] compared 12.5 mg cytoproterone acetate daily to a continuous monophasic OCP once daily (0.02 μg ethinyl estradiol and 0.15 mg desogestrel). The primary endpoint, as in their previous study, was the degree of satisfaction at the end of therapy. A change in severity of symptoms was also measured by a 100 mm visual analogue score and a 0–3 point verbal rating scale. Cyproterone however, has significant anti-androgenic effects.
Levonorgestrel Intrauterine System (LNG-IUS)
The LNG-IUS is a contraceptive intrauterine device (IUD). As it releases norgesterel in a constant fashion, it lessens the excess bleeding associated with other IUD’s, and may even lead to amenorrhea. The LNG-IUS is therefore indicated for women with menorrhagia and dysfunctional bleeding. The constant release of levonorgestrel leads to mean plasma concentrations between 100 and 200 pg/ml [69]. Endometrial exposure to LNG induces endometrial atrophy. Hence the LNG-IUS can only be used for endometriosis in women who do not desire fertility, and who are prepared to accept amenorrhea.
Several small RCTs have compared the use of LNG-IUS in endometriosis to GnRH agonists and Depot medroxyprogesterone acetate [70]. The mechanism by which the LNG-IUS decreases endometriosis related symptoms is unclear, as the LNG-IUS does not inhibit ovulation nor does it induce a hypoestrogenic state. It has been suggested that the LNG-IUS acts by decreasing the expression of glandular and stromal estrogen and progesterone receptors in the ectopic endometrium [71]. With the LNG-IUS a reduction of the severity of endometriosis has been seen at laparoscopy [72, 73]. The echographic size of recto-vaginal lesions under has also been seen on ultrasound under LNG-IUS treatment [74]. The LNG-IUS has been shown to reduce pain [75]. Tanmahasamut [76] compared treatment with the LNS-IUS after laparoscopic conservative surgery to expectant management. There was a significant reduction in dysmenorrhoea [5.0 cm vs 8.1 cm on the visual analogue scale (VAS)] and non-cyclic pelvic pain (VAS 2.2 cm vs 4.8 cm) but no effect on dyspareunia. The LNG-IUS is as effective as DMPA with no impact on bone mineral density [77]. Bayoglu et al. [78] compared the efficacy of the LNG-IUS with the GnRH analogue gosareline on endometriosis related chronic pelvic pain in patients with severe endometriosis during 12 months. Both treatment modalities showed comparable effectiveness in the treatment of chronic pelvic pain related endometriosis.
Norethindrone Acetate (NETA)
Norethindrone (Norethisterone) acetate can be used continuously in a dose of 2.5 mg per day. The evidence for pain relief comes from a study by Muneyyirci-Delale and Karacan [79]. Fifty-two women with endometriosis confirmed by laparoscopy were treated with NETA. Dysmenorrhea and noncyclic pelvic pain were relieved in 48/52 (92.3 %) and 25/28 (89.2 %) of patients, respectively. Similar results were found in recurrent endometrioma [80], and adenomyosis. The advantages of NETA include, excellent cycle control, and no harmful effect on the lipoprotein profile [81]. Ferrero et al. [82] has shown that NETA can relieve the pain of endometriosis, particularly when that pain presents as dysmenorrhea.
Effect of Progestogens on Endometriosis Related Infertility
Numerous mechanisms have been proposed to explain the effect of rndometriosis in infertility:- altered folliculogenesis, reduced preovulatory steroidogenesis by granulosa cells, decreased capability etc. decreased capability of fimbrial ovum capture, sperm phagocytosis by peritoneal and oviductal macrophages, anti-sperm antibodies and reduced sperm penetration and velocity In addition, altered egg–sperm interaction, defective implantation and impaired early embryonic development have been reported to explain endometriosis related infertility. Consequently it is not surprising that although medical management improves the quality of life for many women with endometriosis, the effect on endometriosis related fertility is not so successful. Many progestational agents inhibit ovulation, precluding their use in patients desiring fertility. A Cochrane database metaanalysis [83] which included 23 trials of over 3,000 women found that pretreatment with ovulation inhibiting agents such as oral contraceptives, progestogens, danazol etc. does not improve long term fecundity, and only delays conception. Pregnancy rates following progestin therapy however, depend on the stage of the disease, and whether medical therapy is an adjunct to surgery. To date, there is no randomized controlled trial that has shown an improvement in fertility after any progestin medication. The situation may be different with progestogens which do not affect ovulation such as dydrogesterone. As stated above, dydrogesterone has been shown to improve symptoms and lead to improvement of the findings at laparoscopy. However, in women with infertility and severe disease, there is little evidence of effect on fecundity. For fertility enhancement, surgical treatment is probably preferable, with assisted reproduction immediately after surgery, prior to the recurrence of disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


