Chapter 34
Preterm Labor and Delivery
Janelle R. Walton MD, William A. Grobman MD, MBA
Chapter Outline
INTERACTIONS BETWEEN TOCOLYTIC THERAPY AND ANESTHESIA
Indications for Anesthesia during and after Tocolytic Therapy
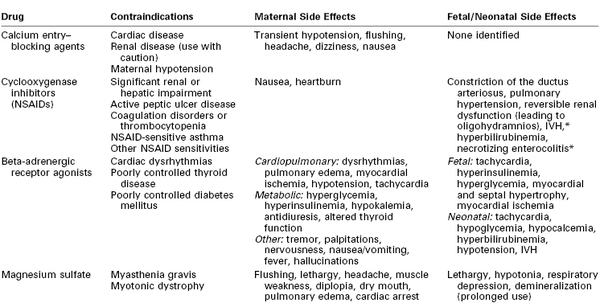
Preterm delivery is defined as delivery before 37 weeks’ gestation. It occurs in 12% to 13% of all pregnancies in the United States and in 5% to 9% of pregnancies in other developed countries.1 Preterm delivery is responsible for 75% to 80% of all neonatal deaths and significant neonatal morbidity.1,2 Birth statistics in the United States reveal a 20% increase in the preterm delivery rate between 1990 and 2006 (from 10.6% to 12.8%) and a 30% increase since 1981.3 Subsequently, the preterm delivery rate declined to 11.72% of all births in 2011 (Figures 34-1 and 34-2).4 Preterm births not only account for a significant degree of neonatal morbidity and mortality but also are responsible for a large economic burden to society. For example, in 2005, the costs associated with preterm birth were at least $26.2 billion.3
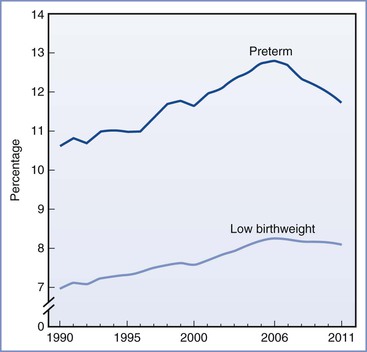
FIGURE 34-1 Preterm and low birth weight rates: United States, final data from 1990 to 2010, preliminary data from 2011. (From Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2011. Natl Vital Stat Rep 2012; 61[5].)
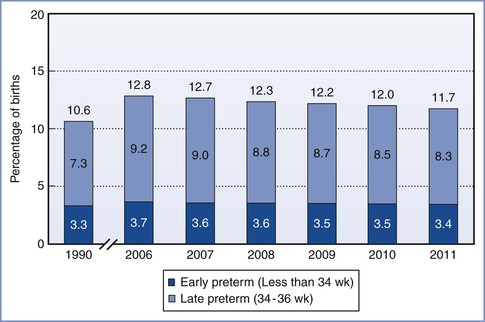
FIGURE 34-2 Total, early, and late preterm birth rates: United States 1990 and 2006 to 2010 (final) and 2011 (preliminary). Preterm birth is defined as less than 37 weeks’ completed gestation. Early preterm is defined as less than 34 weeks’ completed gestation. Late preterm is defined as 34 to 36 completed weeks’ gestation. (Source: Centers for Disease Control and Prevention/NCHS, National Vital Statistics System; natality.)
In 2010, late preterm deliveries comprised 71% of all preterm births (see Figure 34-2). There is a notable racial/ethnic disparity in the frequency of preterm birth. In 2010, 10.8% of non-Hispanic whites, 17.1% of non-Hispanic blacks, and 11.8% of Hispanics delivered preterm. Women age 25 to 34 years were least likely to deliver preterm (11.4%), whereas women 40 years or older were most likely to deliver preterm (25.9%).5
The concern about preterm birth is not confined to the United States; the World Health Organization (WHO) and other nongovernmental organizations have identified the frequency of preterm birth as a critical health issue. The WHO uses low birth weight as an indicator of early delivery because true gestational age at delivery is often not available (see Figure 34-1). Worldwide, the incidence of low birth weight, defined as a birth weight less than 2500 g, is 15.5 per 1000 births.6 Africa and Asia have the highest incidence of low birth weight, at 14.3 and 18.3 per 1000, respectively. In contrast, in Europe and North America the incidence of low birth weight is 6.4 and 7.7 per 1000, respectively.6 Likewise, infant mortality rates are significantly higher in developing nations, particularly the sub-Saharan African countries. The United States has a higher infant mortality rate than Europe (6 versus 3 per 1000 births, respectively), which reflects the higher preterm birth rate in the United States.7
In 2006, the Institute of Medicine recommended that investigators focus on (1) better defining the problem; (2) developing treatments to prevent both preterm delivery and morbidity for children born preterm; (3) identifying the causes of preterm birth, including modifiable risk factors and the reasons for disparity among different ethnic, racial, and socioeconomic groups; and (4) developing policies and public programs that can be used to reduce the rate of preterm birth.3
Definitions
A preterm infant is defined as one who is born between 20 0/7 weeks and 36 6/7 weeks, inclusive, after the first day of the last menstrual period. If a good basis does not exist for establishing the gestational age from maternal history, the exact gestational age is difficult to determine. A low birth weight does not necessarily signify that a neonate has been born preterm, because some newborns have a low birth weight because they are small for gestational age (SGA) rather than preterm. A neonate who weighs less than 2500 g at birth is considered to have a low birth weight (LBW), regardless of gestational age. Likewise, an infant who weighs less than 1500 g at birth is considered to have a very low birth weight (VLBW), and an infant who weighs less than 1000 g at birth is considered to have an extremely low birth weight (ELBW).
Neonatal Mortality
The survival rate among neonates increases as the birth weight and/or gestational age increases (Figure 34-3; Table 34-1).8 After data are controlled for gestational age and weight, male infants have a higher mortality than female infants.9 During the past three decades, there has been a significant improvement in the survival rate for preterm infants, with the greatest improvement occurring in the subgroup with a birth weight between 501 and 1250 g.10 The rate of neonatal survival now exceeds 90% for infants born after 30 weeks’ gestation, and a neonatal survival rate close to that of a term infant can be expected for those born after 32 weeks’ gestation.
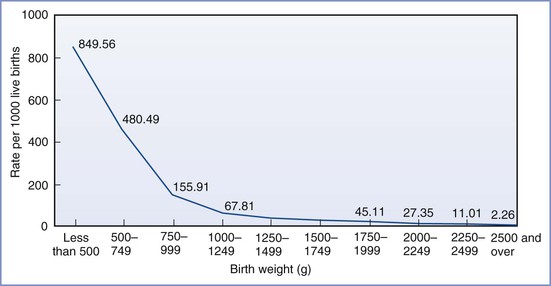
FIGURE 34-3 Infant mortality rates by birth weight: United States, 2004. (From Mathews TJ, MacDorman MF. Infant mortality statistics from the 2004 period linked birth/infant death data set. Natl Vital Stat Rep 2007; 55:1-32.)
TABLE 34-1
Neonatal Deaths by Gestational Age
| Completed Weeks’ Gestation | Percentage of Deaths* |
| 22 | 94 |
| 23 | 74 |
| 24 | 45 |
| 25 | 28 |
| 26 | 16 |
| 27 | 12 |
| 28 | 8 |
* Death rate before discharge by gestational age among infants born in the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network Centers between 2003 and 2007.
Data from Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126:443-56.
However, despite this improvement, infants with a birth weight between 501 and 750 g continue to have high mortality rates (Table 34-2). For example, in a cohort of neonates delivered between 2000 and 2009, the mortality rate for infants weighing between 501 and 1500 g decreased from 14.3% to 12.4%.10 When stratified by birth weight, mortality ranged from 36.6% for infants who weighed between 501 and 750 g to 3.5% for infants who weighed between 1251 and 1500 g.10 A retrospective cohort study assessed survival rates for infants delivered between 24 and 26 weeks’ gestation.11 Neonatal survival was 43%, 74%, and 83% at 24, 25, and 26 weeks’ gestation, respectively. The majority of women received antenatal corticosteroids, and the majority of neonates received exogenous surfactant. A delay in delivery of even 1 week at this time in gestation leads to significantly better outcome and reduced cost. A similar study that examined a cohort of infants born between 1998 and 2002 showed a survival rate of 0% for infants born at 21 completed weeks’ gestation; the survival rate rose steadily to 75% at 25 completed weeks’ gestation (see Table 34-1).12
TABLE 34-2
Selected Outcomes for Extremely Preterm Infants*
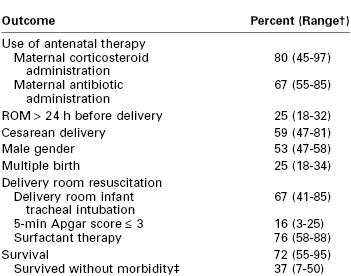
* Birth weight < 1000 g. Data are from the Eunice Kennedy Shriver National Institute of Child Health Development Neonatal Research Network for 9575 infants, gestational ages 22 to 28 weeks, birth weight 401 to 1500 g, between 2003 and 2007.
† Ranges are across 19 Neonatal Research Network centers.
‡ Morbidities included severe intraventricular hemorrhage, periventricular leukomalacia, bronchopulmonary dysplasia, necrotizing enterocolitis, infections, and retinopathy of prematurity (ROP) stage ≥ 3.
ROM, rupture of membranes.
Data from Stoll BJ, Nansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126:443-56.
Infants born at the threshold of viability (22 to 24 weeks’ gestation) continue to have the greatest risk for poor outcome. A recent study examined outcome for infants born between 22 and 27 weeks’ gestation between the years 2002 and 2008. The mortality rate for those born between 22 and 24 weeks’ gestation was 61% compared with 19% for those born between 25 and 27 weeks’ gestation.13 Compared with infants born at 22 to 24 weeks’ gestation, infants born at 25 to 27 weeks’ gestation were more likely to have been exposed to antenatal maternal corticosteroid administration (87% versus 62%), to be delivered by cesarean (70% versus 39%), and to be resuscitated at birth (99% versus 75%). It is estimated that 2% to 5% of VLBW infants who survive to hospital discharge die within 2 years because of medical complications of prematurity.13
Neonatal Morbidity
Approximately 90% of preterm births occur between 32 and 36 6/7 weeks’ gestation. Compared with earlier gestational ages, mortality is less common, but morbidity is a relatively greater concern in this gestational age range. As with mortality, most morbidity decreases in frequency as the gestational age increases. For example, the incidence of high-grade (III or IV) intraventricular hemorrhage (IVH) diminishes rapidly after 27 weeks’ gestation and grade III or IV intraventricular hemorrhages are very rare after 32 weeks’ gestation. Likewise, neonatal morbidity from patent ductus arteriosus and necrotizing enterocolitis diminishes significantly after 32 weeks’ gestation.12 Data from the National Institute of Child Health and Development (NICHD) Neonatal Research Network sites from 1997 through 2002 indicate that survival without complications (e.g., bronchopulmonary dysplasia, severe intraventricular hemorrhage, necrotizing enterocolitis, or a combination of these disorders) ranged from 20% for infants with a birth weight between 501 and 750 g to 89% for those with a birth weight between 1251 and 1500 g.2
Piecuch et al.14 reported data for a cohort of 138 nonanomalous infants delivered between 24 and 26 weeks’ gestation between 1990 and 1994. The incidence of cerebral palsy did not differ significantly among the three groups born at 24, 25, and 26 weeks’ gestation (11%, 20%, and 11%, respectively). However, the incidence of normal cognitive outcome rose with increasing gestational age at birth (28%, 47%, and 71% at 24, 25, and 26 weeks’ gestation, respectively).
The EPICure study group assessed the association between extreme preterm delivery and long-term physical and mental disability in a cohort of infants delivered between 22 and 25 weeks’ gestation during a 10-month period in 1995.15 These investigators noted rates of severe disability of 54%, 52%, and 45% among infants delivered at 23, 24, and 25 weeks’ gestation, respectively. In a later cohort of infants, born between 1997 and 2002, the rates of severe disability were 33%, 21%, and 12% for infants delivered at 23, 24, and 25 weeks’ gestation, respectively.16 A 6-year follow-up to the EPICure study cohort showed persistent severe disability in 25%, 29%, and 18% of infants born at 23, 24, and 25 weeks’ gestation, respectively.17
Hack et al.18 monitored a cohort of ELBW infants born between 1992 and 1995 until they were 8 years old. The mean birth weight was 810 g, and the mean gestational age at delivery was 26 weeks. Compared with a cohort of age-matched children of normal birth weight, the ELBW group had a higher incidence of significant neurosensory impairment (16% versus 0%, respectively) and asthma (21% versus 9%). The ELBW children differed significantly from the cohort with normal birth weight in rates of suboptimal intelligence, academic achievement, motor skills, and adaptive functioning. These data illustrate the long-term medical, educational, and social services required by these children.
Correspondingly, the economic costs for the care of surviving preterm infants (especially VLBW infants) can be enormous. The Institute of Medicine estimated that the societal economic burden associated with preterm birth in the United States was at least $26.2 billion in 2005, or $51,600 per infant born preterm.3 These figures likely will continue to rise with the escalating cost of health care.
Preterm Labor
Risk Factors
Box 34-1 lists factors associated with preterm labor.1,19,20 These associations do not necessarily indicate cause-and-effect relationships. Significant risk factors include a history of preterm delivery, non-Hispanic black race (irrespective of socioeconomic status), and multiple gestation.
The process of normal parturition involves anatomic, physiologic, and biochemical changes that lead to (1) greater uterine contractility, (2) cervical ripening, and (3) membrane/decidual activation.19,20 The fetus also appears to play a role in parturition. It is hypothesized that the mature fetal hypothalamus secretes more corticotropin-releasing hormone (CRH), which in turn stimulates fetal adrenal production of adrenocorticotropic hormone (ACTH) and cortisol.19 Preterm labor results from the pathologic activation of one or more of these components (Figure 34-4).21 Preterm delivery results from (1) preterm premature rupture of membranes (preterm PROM) in approximately 30% of cases, (2) spontaneous preterm labor in approximately 45% of cases, and (3) maternal or fetal indications for early delivery in approximately 25% of cases.22 However, the “spontaneous” causes do not have a uniform underlying pathophysiology, and it appears that preterm labor is a syndrome with multiple causes influenced by a number of genetic, biologic, biophysical, psychosocial, and environmental factors.
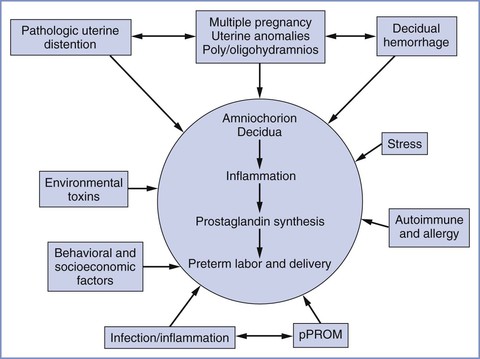
FIGURE 34-4 Major etiologic factors in preterm birth, including activation of the maternal or fetal hypothalamic-pituitary axis (stress), inflammation, decidual hemorrhage, and pathologic distention of the myometrium. The pathways are not mutually exclusive and may overlap, and they share a common biochemical pathway. pPROM, preterm premature rupture of membranes. (From Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand 2008; 87:590-600.)
Two emerging factors of interest are the influences of infection and uterine distention on initiation of myometrial contractility. Infection is thought to be present in up to 40% of preterm deliveries.1 Commonly identified organisms include Ureaplasma urealyticum, Bacteroides species, Neisseria gonorrhoeae, Chlamydia trachomatis, group B streptococci, Staphylococcus aureus, Treponema pallidum, and enteropharyngeal bacteria.23–25 Although approximately 50% of preterm deliveries occur in women with no apparent risk factors, subclinical infection may precipitate preterm labor in some of these cases.24
In the past three decades there has been a significant rise in the incidence of multiple gestation (see Chapter 35). The twin birth rate increased by 76% from 1980 to 2009.5 The triplet and higher-order multiple birth rate rose by over 400% during the 1980s and 1990s but has declined 29% since the 1998 peak.4 The increased incidence of multiple gestation is due, in part, to the significant increase in the use of assisted reproductive technologies (ARTs).26 Over 50% of all twins and 90% of all triplets are born preterm.5 Consequently, multiple gestations account for 17% of all preterm births.27 ART pregnancies are also associated with an increase in risk for preterm delivery, even for singleton pregnancies.28 A 2004 meta-analysis of 15 studies, which compared outcomes for 12,283 ART singleton pregnancies with outcomes for 1.9 million spontaneously conceived singleton pregnancies, demonstrated a higher risk for preterm and SGA deliveries in the ART group.28 Placenta previa, gestational diabetes, preeclampsia, and neonatal intensive care unit admission were also more prevalent in the ART group.28
Approximately 25% of all preterm deliveries do not result from spontaneous preterm labor or preterm PROM. The obstetrician may choose to perform indicated delivery for maternal or fetal indications, such as severe preeclampsia or a nonreassuring fetal status.
Prediction of Preterm Labor
The ability to prevent spontaneous preterm birth would be facilitated if it were possible to intervene prophylactically to prevent preterm labor or to effectively treat preterm labor once it occurs. Both prophylaxis and treatment would be facilitated if it were possible to accurately predict which asymptomatic or symptomatic patients would have spontaneous preterm delivery. Several methods of predicting preterm delivery have been proposed, including home uterine activity monitoring, salivary estriol measurement, fetal fibronectin screening, and transvaginal cervical ultrasonography.29
The use of home uterine activity monitoring to identify women at risk for preterm labor has been investigated in a number of randomized controlled trials.29 Likewise, salivary estriol levels have been assessed as a marker of risk for preterm delivery. Neither has been shown to be clinically useful in the prevention of preterm birth.
Fetal fibronectin (fFN) is a basement membrane glycoprotein produced by the fetal membranes. It functions as an adhesive protein of the placental membranes to the decidua.30 fFN is normally absent from vaginal secretions from 20 weeks’ gestation until near term. Detection of elevated levels of fFN is associated with an increased risk for preterm delivery. It is hypothesized that the presence of fFN is a marker of choriodecidual disruption. A study from the Maternal-Fetal Medicine Units Network documented that a positive fFN test result at 22 to 24 weeks’ gestation had a sensitivity of 63% in predicting preterm labor before 28 weeks’ gestation.31 If fFN is absent (i.e., a negative result), the risk for preterm delivery within 1 or 2 weeks is less than 1%.32 The high negative predictive value of this test may make it a useful tool to help triage symptomatic patients more efficiently, although this remains to be demonstrated in randomized trials33; currently, there is no role for this test in screening low-risk asymptomatic women.29
Short cervical length, as assessed by transvaginal ultrasonography, also is associated with a greater risk for preterm delivery.34 In a 2006 systematic analysis, Kagan et al.35 concluded that cervical length is associated with preterm delivery (i.e., the shorter the cervix the greater the risk for preterm delivery) in symptomatic women. Further, multiple studies have shown an increased risk for preterm delivery in asymptomatic women with a shortened cervix.29 A Maternal-Fetal Medicine Units Network study of nearly 3000 women found that the risk for spontaneous preterm delivery is increased in women with evidence of a short cervix detected by transvaginal ultrasonography between 24 and 28 weeks’ gestation. A cervical length below the 10th percentile had a sensitivity of 37% and a specificity of 92% in predicting preterm birth before 35 weeks’ gestation, with a corresponding positive predictive value of 18% and a negative predictive value of 97%.34
A history of cervical surgery, including conization and loop electrosurgical excision procedure, traditionally has been thought to be a risk factor for preterm birth because of associated cervical injury. However, this relationship may be related to environmental factors and/or behavioral factors that underlie the progression of cervical dysplasia. Uterine instrumentation, such as dilation and curettage, also has been associated with an increased risk for preterm birth in some, but not all, studies; the mechanism is unclear, but it may be a result of intrauterine microbial colonization, injury to the endometrium, or both, together with host and environmental factors.29
Prevention of Preterm Labor
Antenatal screening for risk factors for preterm labor and delivery is of value only if interventions are available that can decrease the frequency of preterm delivery and improve neonatal outcome.29 Unfortunately, few if any interventions have been shown to definitively reduce these outcomes. Interventions that have been studied include detection and suppression of uterine contractions, antimicrobial therapy, prophylactic cervical cerclage, maternal nutritional supplements, and reduction of maternal stress.20 It is not surprising that most of these simple interventions have not been shown to alter outcome, given that preterm labor is increasingly understood to be a complex syndrome with multiple, overlapping causes.20
Prophylactic cervical cerclage in the early second trimester has been performed to prevent preterm birth, typically in women with a history of mid-trimester pregnancy loss. Evidence that supports the efficacy of this practice is weak.36 There remains controversy with regard to whether cerclage should be placed in response to transvaginal ultrasonographic evidence of a short cervix in the second half of the mid trimester. Data do not support such a practice in a general population,37 but there is some evidence that such a practice may be beneficial among high-risk women, such as those with a prior preterm birth.38
Evidence does not support the administration of prophylactic antibiotics in asymptomatic women at risk for preterm labor.39 Likewise, evidence does not support the prophylactic use of beta-adrenergic receptor agonists to prevent preterm labor in high-risk women.40
In contrast, evidence suggests that progesterone therapy may be effective in reducing the rate of preterm birth in some patient populations. The Maternal-Fetal Medicine Units Network performed a randomized controlled trial that compared prophylactic intramuscular 17α-hydroxyprogesterone caproate (17P) (250 mg weekly beginning at 16 to 20 weeks’ gestation, and continued until delivery or 36 weeks’ gestation) with placebo in women with a history of spontaneous preterm delivery.41 The risk for delivery before 37 weeks’ gestation was reduced in the 17P group (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54 to 0.81). Similarly, da Fonseca et al.42 demonstrated a decreased risk for preterm delivery (< 34 weeks’ gestation) in high-risk women randomly assigned to receive either vaginal progesterone (100 mg daily) or placebo. A systematic review of 11 randomized controlled trials (n = 2425) also concluded that progesterone administration was associated with a significant reduction in recurrent preterm birth in women with a history of spontaneous preterm delivery.43
Progesterone therapy also has been shown to be efficacious in preventing preterm delivery among women with a short cervix identified by transvaginal ultrasonography. In two double-blind, placebo-controlled trials, women with a mid-trimester diagnosis of a short cervix (< 15 mm in one trial44 and 10 to 20 mm in the other45) were randomized to receive either vaginal progesterone or placebo. Women who received vaginal progesterone experienced a significant reduction in the frequency of preterm delivery before 33 weeks’ gestation.44,45 In contrast, in a trial that enrolled nulliparous women with a cervical length less than 30 mm, women randomized to receive 17P did not experience a reduction in preterm delivery compared with women who received placebo.46
A number of studies have examined whether progesterone is efficacious in reducing preterm birth among women with multiple gestation. Uniformly, progesterone therapy has not been shown to be beneficial in this population.47–49
Even among women for whom progesterone is thought to be indicated, the optimal type, timing, and dosing of progesterone is unclear. Based on existing evidence, the American College of Obstetricians and Gynecologists (ACOG)29 has concluded that vaginal progesterone should be offered to asymptomatic women with a singleton gestation without a previous preterm delivery, who have a very short cervical length (i.e., ≤ 20 mm at or before 24 weeks’ gestation).
Diagnosis
Determining whether a woman is in early preterm labor or in false labor is often difficult. Criteria for the diagnosis of preterm labor include gestational age between 20 0/7 and 36 6/7 weeks’ gestation and regular uterine contractions accompanied by a change in cervical dilation, effacement, or both (or initial presentation with regular contractions and cervical dilation of 2 cm or more).19 Less than 10% of women with the clinical diagnosis of preterm labor actually give birth within 7 days of presentation.19
Assessment and Therapy
Initial assessment of the patient with possible preterm labor includes physical examination and external monitoring of contractions with a tocodynometer (and fetal heart rate if indicated by the gestational age). Acute conditions associated with preterm labor should be considered, including infection and placental abruption. Maternal physical examination may include a sterile speculum examination to exclude preterm PROM if symptoms or signs indicate this possibility. In many women who have preterm uterine contractions, these contractions will cease spontaneously. In the past, clinicians assumed that intravenous hydration was a useful component of therapy. However, there is no evidence that intravenous hydration reduces the chance of preterm delivery.50
Once the diagnosis of preterm labor is established, the obstetric care provider must decide whether intervention is warranted. The therapeutic agents currently thought to be associated with improved neonatal outcomes include antenatal maternal corticosteroid administration to accelerate maturation of fetal lungs and other developing organ systems, and the targeted use of magnesium sulfate for fetal neuroprotection (see later discussion).51,52 Although widely used before 34 weeks’ gestation, acute tocolytic therapy remains a source of controversy. There is no consistent evidence that the use of acute tocolysis reduces the chance of preterm birth or improves neonatal outcome. However, because acute tocolysis has been associated with a short (approximately 48 hour) prolongation of pregnancy, it may be used to facilitate transfer of the patient from a community hospital to a tertiary care facility that can provide optimal care for the preterm neonate. Moreover, a short course of tocolytic therapy may delay delivery for 24 to 48 hours, allowing maternal administration of (1) a corticosteroid to accelerate fetal lung maturity and (2) antibiotic therapy to prevent neonatal group B streptococcal infection. Thus, the ACOG has supported the use of acute tocolysis to allow administration of a complete course of antenatal corticosteroids, but the ACOG discourages the continued use of tocolysis after corticosteroid administration is complete.19
Criteria for the use of tocolytic therapy include (1) gestational age after viability and before 34 weeks’ gestation, (2) reassuring fetal status, and (3) no overt clinical signs of infection. The potential benefits of delaying delivery of the preterm infant (i.e., decreased neonatal morbidity and mortality) must be weighed against the maternal and fetal risks (e.g., maternal side effects of tocolytic drugs, deterioration of a compromised fetus). Box 34-2 lists contraindications to the inhibition of labor.
In the setting of preterm PROM, obstetricians have worried that tocolytic therapy might increase the risk for maternal and/or fetal infection. It also seems logical that tocolytic therapy is less effective in patients with preterm PROM. Prospective, randomized studies have shown that tocolytic therapy does not improve neonatal outcome compared with conservative expectant management in patients with preterm PROM.53
Antenatal Administration of Corticosteroids
The neonatal benefits of corticosteroid administration (Table 34-3) before preterm delivery have been clearly demonstrated in large clinical trials. The NICHD Neonatal Research Network evaluated outcomes for 11,718 preterm infants delivered after antenatal maternal corticosteroid administration between 1988 and 1992. Antenatal corticosteroid treatment significantly reduced the incidence of neonatal respiratory distress syndrome, intraventricular hemorrhage, and neonatal death in all subgroups of the population studied (including male and female infants, African and Caucasian race infants, and infants delivered before 30 weeks’ gestation).54 The reduction in neonatal morbidity and mortality from antenatal corticosteroid administration is additive to the reduction observed with the use of neonatal surfactant alone.55
TABLE 34-3
Antenatal Corticosteroid Therapy
| Drug | Dose and Route | Frequency/Duration |
| Betamethasone | 12 mg IM | Every 24 h × 2 |
| Dexamethasone | 6 mg IM | Every 12 h × 4 |
IM, intramuscular.
From National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: Repeat courses—National Institutes of Health Consensus Development Conference Statement, August 17-18, 2000. Obstet Gynecol 2001; 98:144-50.
Although there is little controversy about the efficacy of a single course of antenatal corticosteroids, there remains debate over the use of multiple courses of corticosteroids for women who remain undelivered 7 days after the initial dose of corticosteroids. A 2001 review56 and a National Institutes of Health (NIH) consensus panel statement57 did not recommend multiple courses of corticosteroids; however, both documents cited some evidence of their possible benefit. These documents also identified possible risks, including a higher incidence of neonatal infection and potentially deleterious effects on neuronal and organ growth.56,57 A large study performed by the Maternal-Fetal Medicine Units Network randomly assigned women at risk for preterm delivery between 23 and 32 weeks’ gestation to receive either a single course or repeated (weekly) courses of antenatal corticosteroids.58 Weekly corticosteroid administration did not significantly reduce the composite primary morbidity outcome, but it significantly reduced the need for neonatal surfactant, mechanical ventilation, and continuous positive airway pressure (CPAP), as well as the incidence of pneumothorax. In contrast, weekly corticosteroid administration was associated with an increase in the delivery of SGA infants, and there was a significant reduction in the birth weight of the infants whose mothers received four or more courses of corticosteroids.
To balance the potential beneficial effects and risks of additional courses of corticosteroids, some have advocated for a single “rescue” course (i.e., a second course of corticosteroids), which is administered at the time of a second episode of preterm labor with a high probability of preterm delivery. One randomized trial demonstrated that additional neonatal benefit could be derived from a single “rescue” course of corticosteroids.59 The investigators reserved this intervention for patients with intact membranes, whose antecedent corticosteroid treatment had been given at least 2 weeks before the “rescue” course, whose gestational age was less than 33 weeks, and who were judged by the clinician as likely to give birth within the next week. A meta-analysis60 concluded that a single “rescue” course of antenatal corticosteroids should be considered in women whose prior course of antenatal corticosteroids was administered at least 7 days previously and who were at acute risk for preterm delivery before 34 weeks’ gestation. The ACOG51 has stated that one “rescue” course of corticosteroids may be considered in these specific populations. However, regularly scheduled repeat courses or multiple courses (more than two) of corticosteroids are not currently recommended.51
Antibiotic Therapy
The results of a large multicenter randomized controlled trial61 and a meta-analysis62 do not support the use of prophylactic antibiotic therapy in the management of preterm labor in patients with intact membranes as a method to reduce the likelihood of preterm birth. In fact, in one study that assessed long-term outcome for offspring born to women who participated in a randomized placebo-controlled trial of antibiotic administration in the setting of preterm labor, children born to women who received antibiotic treatment had more functional health impairment.63 Accordingly, the ACOG19 does not recommend empirical antibiotic therapy in this population. It should be noted, however, that prophylactic antibiotic administration remains appropriate in women who are positive for group B streptococcus (GBS) and who are thought to be in preterm labor.64
In contrast, in patients with preterm PROM, randomized controlled trials and a meta-analysis have concluded that antimicrobial therapy prolongs pregnancy and reduces both maternal and neonatal morbidity.65,66 Thus, when preterm PROM is diagnosed, the ACOG67 recommends a 7-day course of antimicrobial therapy; the best antibiotic regimen is not known with certainty, although intravenous ampicillin and erythromycin (48 hours), followed by oral amoxicillin and erythromycin (5 days), is a commonly used regimen for women with preterm PROM who are receiving expectant management.67
Neuroprotection
Several clinical trials have provided evidence that maternal administration of magnesium sulfate provides fetal neuroprotection when given to women at risk for preterm delivery. In 2003, Crowther et al.68 reported the results of a multicenter randomized, placebo-controlled study of 1062 women (1255 infants) at less than 30 weeks’ gestation, in whom delivery was planned or expected within 24 hours. The investigators observed no significant difference between groups in the primary outcomes, which included total pediatric mortality, cerebral palsy, or both, at a corrected age of 2 years. However, they observed a significantly reduced rate of substantial gross motor dysfunction, as well as a reduced combined rate of death or substantial gross motor dysfunction, in the children exposed to magnesium sulfate in utero.68 Similarly, a randomized controlled trial of magnesium sulfate administration in 573 pregnant women at less than 33 weeks’ gestation, and in whom delivery was planned or expected within 24 hours, found that infants exposed to magnesium sulfate had a reduced rate of total neonatal mortality, severe cerebral white matter injury (which is associated with cerebral palsy), and the combination of severe white matter injury and/or death, but the differences were not statistically significant.69 In the largest randomized trial,70 2241 women at imminent risk for delivery before 32 weeks’ gestation were randomized to receive magnesium sulfate or placebo. The offspring who had been exposed to magnesium sulfate in utero were significantly less likely to develop moderate/severe cerebral palsy (1.9% versus 3.5%; RR, 0.55; 95% CI, 0.32 to 0.95).70
A recent meta-analysis has synthesized the data from the clinical trials and suggests that prenatal administration of magnesium sulfate reduces the occurrence of cerebral palsy when given with neuroprotective intent (RR, 0.71; 95% CI, 0.55 to 0.91).71 The ACOG52 recently stated that, based on available evidence, magnesium sulfate—given before anticipated early preterm birth—reduces the risk for cerebral palsy in surviving infants. Because the best regimen of magnesium sulfate administration remains unclear, physicians electing to use magnesium sulfate for fetal neuroprotection should develop specific guidelines regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring based on the protocols of the larger published trials.52
Selection of Tocolytic Agents
Once the obstetrician has decided to begin tocolytic therapy, an appropriate agent must be selected (Table 34-4). (Each specific class of tocolytic agent is discussed in detail later in this chapter.) A 2003 analysis of studies that compared the four classes of tocolytic agents currently in use (i.e., beta-adrenergic receptor agonists, calcium entry–blocking agents, magnesium sulfate, and nonsteroidal anti-inflammatory drugs [NSAIDs]) concluded that all are more effective than placebo in prolonging pregnancy, but the investigators found no evidence of a beneficial effect on neonatal morbidity or mortality.72 A more recent analysis suggested that magnesium sulfate specifically is not efficacious and should not be used for tocolysis.73
Physiology of Uterine Contractions
The contractile elements in myometrial smooth muscle consist of thick (myosin) and thin (actin) filaments that interact and slide past one another, generating the contractile force for uterine contractions. The myometrium has pacemaker cells with spontaneous contractile ability, which spread activity throughout the rest of the uterus by means of gap junctions between myometrial cells. Myometrial contractions are preceded by a rise in intracellular calcium concentration through the influx of calcium across the sarcolemma and/or release from internal stores such as the sarcoplasmic reticulum. Hormones and neurotransmitters may play a role in the regulation of uterine activity by causing agonist-induced entry of calcium or other ions by means of receptor-controlled channels and the release of calcium from internal stores.74
The rise in intracellular calcium results in the formation of a complex between calcium and calmodulin (a regulatory enzyme), which activates myosin light-chain kinase (MLCK). Activated MLCK then phosphorylates the light-chain subunit of myosin, allowing actin to bind to myosin and activate myosin adenosine triphosphatase. Adenosine triphosphate (ATP) is then hydrolyzed, and muscle shortening or contraction results. Relaxation of smooth muscle results from a reduction in the intracellular calcium concentration and/or dephosphorylation of the myosin light chain by myosin light-chain phosphatase. Increases in intracellular cyclic adenosine monophosphate (cAMP) also can result in muscle relaxation by two mechanisms: (1) activation of a cAMP-dependent protein kinase, which decreases the activity of MLCK; and (2) a reduction of the intracellular calcium concentration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree